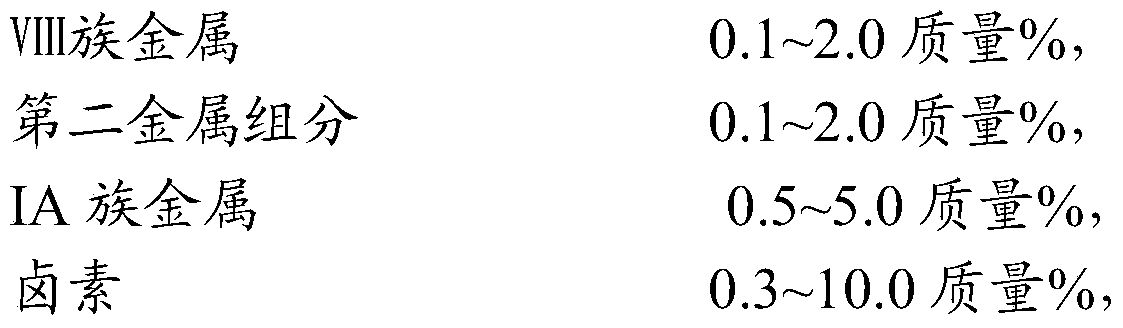A kind of low-carbon alkane dehydrogenation olefin catalyst and preparation method thereof
A catalyst and low carbon alkane technology, applied in the direction of hydrocarbons, hydrocarbons, chemical instruments and methods, etc., can solve the problems of increased propane cracking reaction, carbon deposition, accelerated catalyst deactivation rate, etc., to achieve improved stability, The effect of high carbon capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
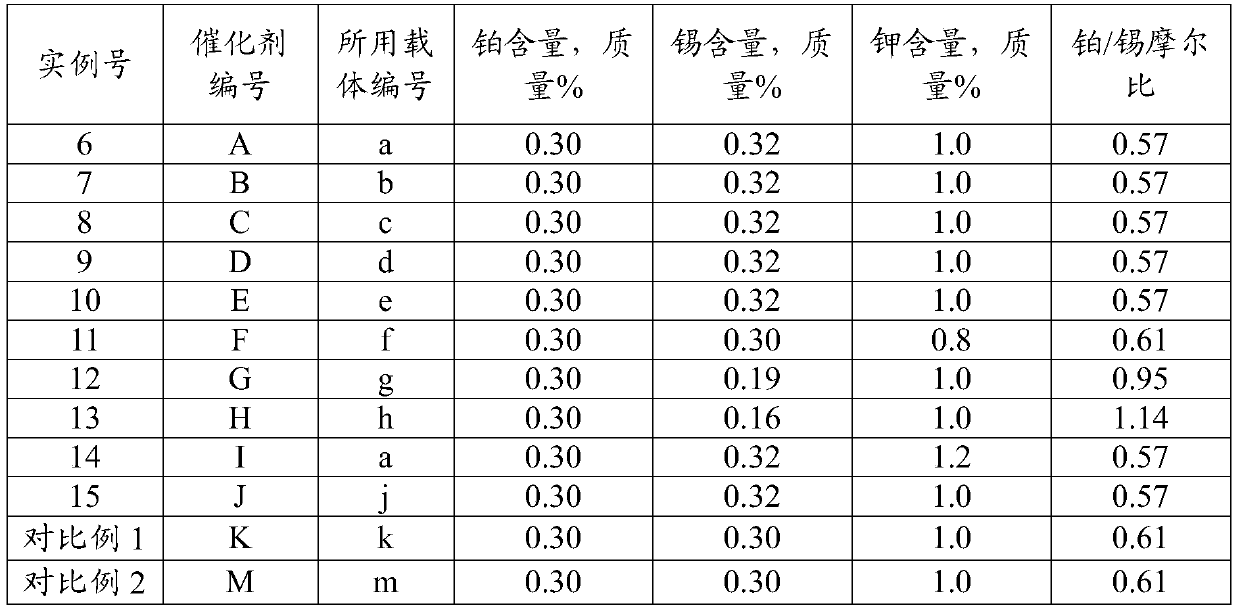
Examples
preparation example Construction
[0020] The preparation method of catalyst provided by the invention comprises the steps:
[0021] (1) adding acid peptization to aluminum hydroxide to obtain alumina sol, adding a pore-enlarging agent and a compound containing the second metal component to the alumina sol, forming a drop ball,
[0022] (2) After drying the wet ball formed by the step drop ball in (1), perform one-stage calcination at 620-680°C, and then heat up to 900-1100°C for two-stage calcination to obtain the carrier.
[0023] (3) impregnating the carrier prepared in step (2) with a solution containing Group VIII metals and halogen-containing compounds, drying, and calcining at 400-650°C,
[0024] (4) impregnating the carrier obtained in step (3) with a water-soluble compound solution containing Group IA metal, drying, and calcining at 400-650°C.
[0025] In the above-mentioned method, (1) step is to form with alumina sol dropping ball, the pore-enlarging agent that adds in alumina sol (aluminum sol) is ...
example 1
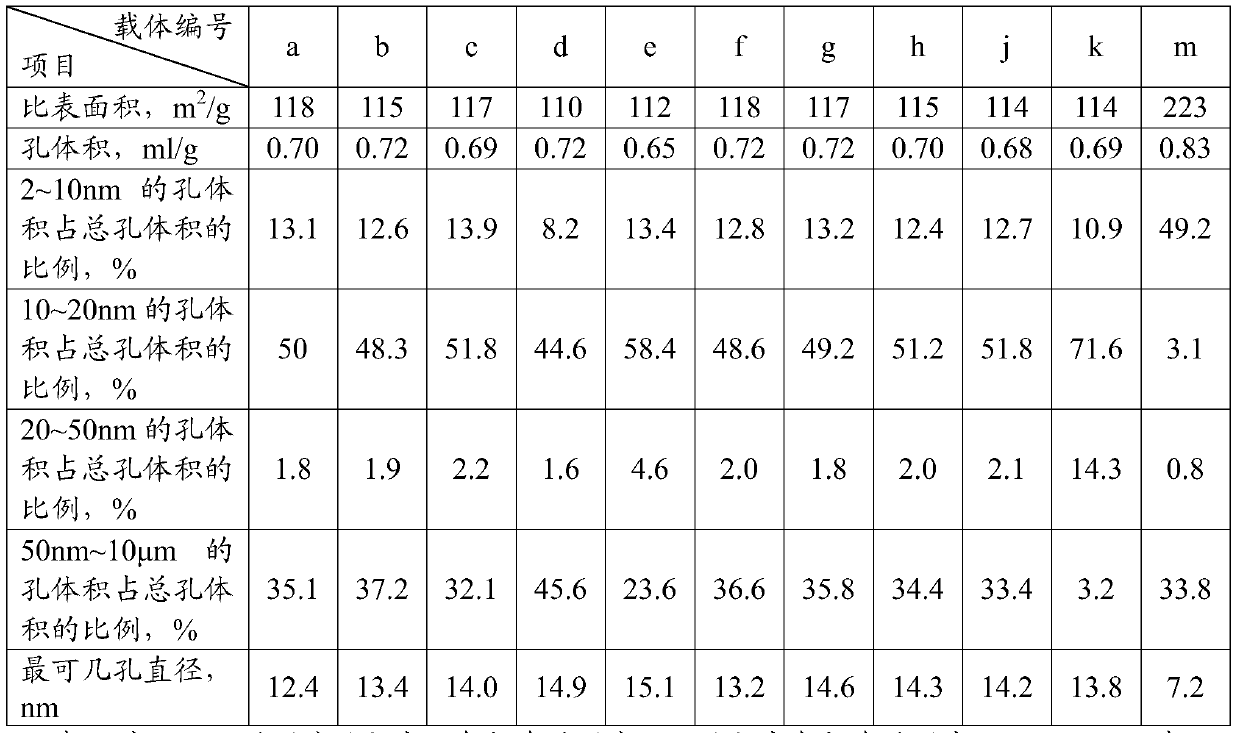
[0043] The alumina support described in the present invention is prepared.
[0044] Take 27 g of aluminum flakes, add 610 g of hydrochloric acid solution with a concentration of 18% by mass to dissolve the aluminum flakes, and obtain a solution with an aluminum trichloride content of 4% by mass. Transfer the aluminum trichloride solution into a neutralization tank, add 850 grams of ammonia water with a concentration of 6% by mass, mix evenly at 60° C., and the pH value is 7.5 to 8.5. The generated aluminum hydroxide was filtered and washed, and 9 mL of nitric acid with a volume ratio of 1:1 was added to the filter cake to acidify to obtain a sol.
[0045] Add 40 mL of a solution containing 30 g of urea and a hydrochloric acid solution containing 32 g of stannous chloride to the sol under stirring, so that the Sn content in the solution is 0.32% by mass of the dry basis alumina, and stir for 1 hour to acidify. Then 30 g of kerosene and 3 g of fatty alcohol ethoxylates were add...
example 2
[0047] The alumina carrier was prepared according to the method of Example 1, except that 132 grams of aluminum hydroxide powder (produced by German Sasol Company, brand SB, with an alumina content of 76% by mass) was taken, and 100 mL of deionized water was added, and stirred for 1 hour to make the slurry Then add 9 mL of nitric acid with a volume ratio of 1:1 to acidify to obtain aluminum sol, and obtain tin-containing θ-Al 2 o 3 Carrier b, its physical properties are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com