Nitrogen-doped carbon-supported non-precious metal (m-n-c) oxygen reduction catalyst and preparation method thereof
A non-precious metal, nitrogen-doped carbon technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve unfavorable catalyst industrialization, difficult control of preparation process conditions, and complex catalyst preparation methods and other problems, to achieve excellent stability and methanol resistance, good ORR catalytic activity, and facilitate the effect of diffusion transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
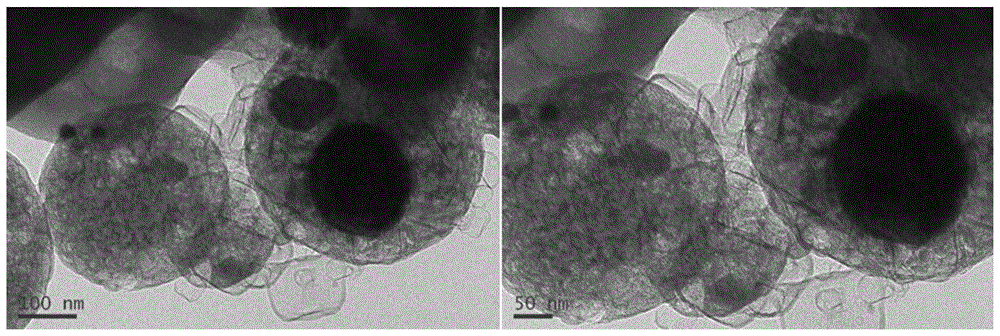
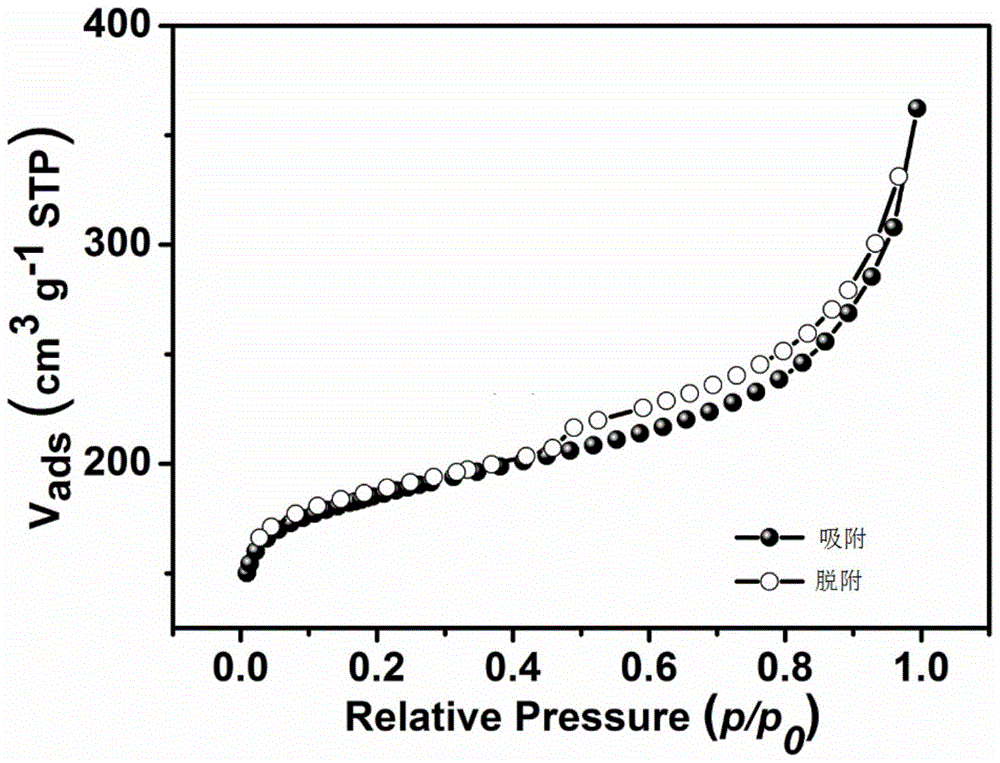
Image
Examples
preparation example
[0054] Synthesis of tripyrrole-[1,3,5]-triazine (TPT):
[0055] Add 6.78g (0.10mol) of pyrrole and 80mL of anhydrous tetrahydrofuran into a 250mL single-necked round-bottomed flask, add KOH (8.86g, 0.15mol) into the above system under ice-cooling, rise to room temperature for 3 hours, and divide Add 5.59g (0.03mol) of cyanuric chloride in batches, and continue stirring at room temperature for 18h. After the reaction was completed, the reaction system was poured into a 250mL ice-water bath to settle, filtered, washed with deionized water three times (200mL×3), and then dried in a vacuum oven at 80°C for 24h. The obtained crude product was recrystallized with 15 mL of acetone and ethanol mixed solvent (acetone: ethanol ratio of 4:1), filtered, washed with ethanol, and the obtained off-white solid was vacuum-dried at room temperature to constant weight, 4.64 g, yield 56%, melting point: 210 ° C .
Embodiment 1
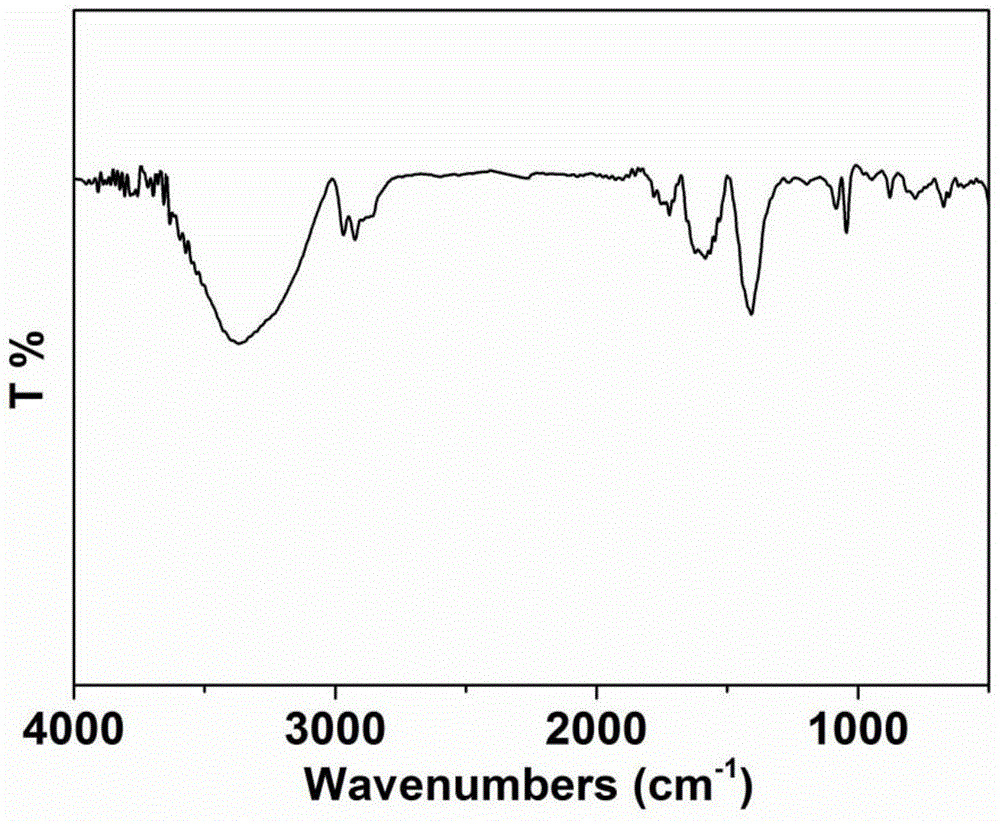
[0057] (1) Synthesis (P(TPT+Py)): Dissolve 55g (0.2mol) tripyrrole-[1,3,5]-triazine (TPT), 40.6g (0.6mol) pyrrole (Py) in 2000mL nitric acid Add 18.26g (0.24mol) of dimethoxymethane and 32.28g (0.24mol) of anhydrous aluminum trichloride to benzene, stir at 45°C for 5h, then rise to 80°C and stir for 19h; after the reaction is complete, pour Sediment in 10L methanol, filter, wash with water (1000mL×2), and dry for later use; the infrared spectrum of polymer P (TPT+Py) is as follows figure 1 shown;
[0058] The partial structure (i.e. repeating unit) of the polymer is as follows:
[0059]
[0060] (2) Add 2.5g (P(TPT+Py)) and 0.7g ferric trichloride to 300mL ethanol, ultrasonically disperse the whole system for 180min, evaporate the ethanol to dryness, and dry in a vacuum oven at 80°C 4h;
[0061] (3) Gained 3.2g powdery solid in N 2 Heat treatment at 800°C for 1 h in the atmosphere to obtain 0.9 g of nitrogen-doped carbon material;
[0062] (4) The obtained nitrogen-dop...
Embodiment 2
[0071] (1) Synthesis (P(TPT+Py)): Dissolve 55g (0.2mol) tripyrrole-[1,3,5]-triazine (TPT), 40.6g (0.6mol) pyrrole (Py) in 2000mL nitric acid Add 24g (0.24mol) dichloroethane and 32.28g (0.24mol) anhydrous aluminum trichloride to benzene, stir at 45°C for 5h, then rise to 80°C and stir for 19h; after the reaction is complete, pour 10L methanol Settled in medium, filtered, washed with water (1000mL×2), and dried for later use; the infrared spectrum of polymer P (TPT+Py) is as follows figure 1 shown;
[0072] (2) Add 2.5g (P(TPT+Py)) and 0.7g ferric trichloride to 300mL ethanol, ultrasonically disperse the whole system for 180min, evaporate the ethanol to dryness, and dry in a vacuum oven at 80°C 4h, obtain primary mixture;
[0073] (3) Gained 3.2g powdery solid in N 2 Heat treatment at 800°C for 1 hour in the atmosphere to obtain 0.91g of nitrogen-doped carbon material;
[0074] (4) The obtained nitrogen-doped carbon material was washed with 2000mL 0.5M dilute sulfuric acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com