Preparation method of carfilzomib key intermediate and derivatives thereof
A carfilzomib and intermediate technology, applied in the direction of organic chemistry and the like, can solve the problems of poor stereoselectivity, low yield and high cost, and achieve the effects of mild operating conditions, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
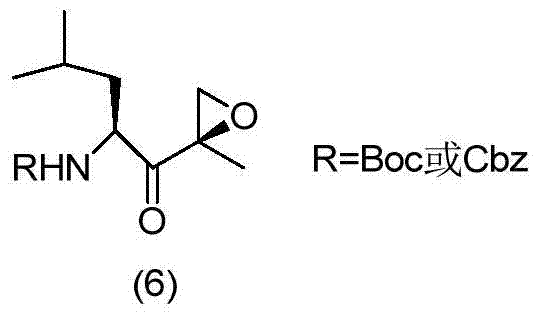
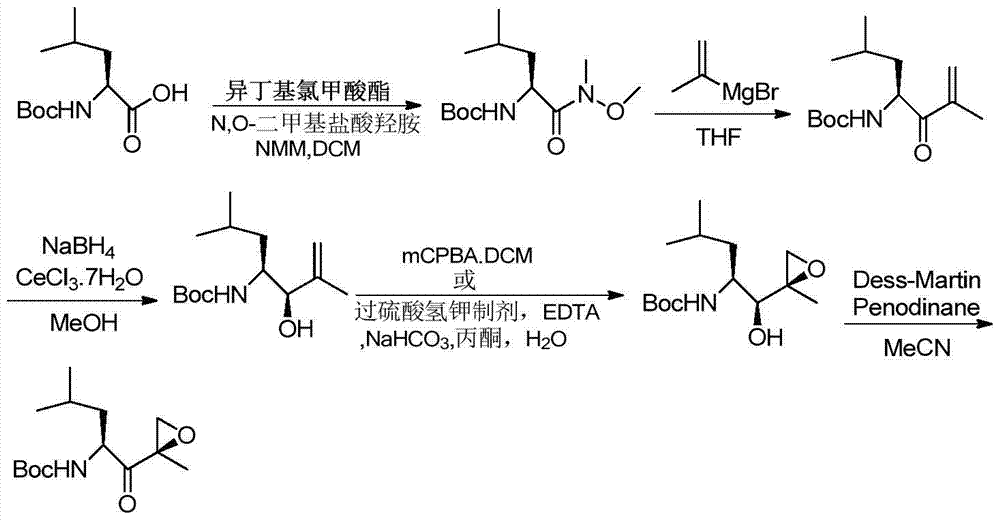
[0052] The following specific examples are used to further illustrate the present invention, but they are not meant to limit the scope of the present invention in any way. Example 1: Synthesis of [(1S)-3-methyl-1-[[(2R)-2-methyloxiranyl]carbonyl]butyl]-carbamic acid tert-butyl ester (6)
[0053]
[0054] Add 0.12mol[(1S,2S)-1-hydroxy-4-methyl-1-[[(2R)-2-methyloxiranyl]pent-2-yl]carbamate (5 ) Dissolve in 100ml dimethyl sulfoxide, add 0.24mol diisopropylethylamine, add 0.24mol pyridine sulfur trioxide in batches under ice bath, warm to room temperature to react, TLC detects the reaction to completion, add appropriate amount of water and use acetic acid The ethyl ester was extracted and washed with 1N dilute hydrochloric acid and saturated brine once, and the organic phase was dried and concentrated to obtain 0.10 mol of the epoxidized product (6) with a yield of 83.3%.
[0055] 1 H NMR(400MHz, CDCl 3 )δ4.86(d,J=8.0Hz,1H), 4.30(t,J=9.6Hz,1H), 3.27(d,J=4.8Hz,1H), 2.87(d,J=4.8Hz,1H) ...
Embodiment 2
[0057] Example 2: Synthesis of [(1S)-3-methyl-1-[[(2R)-2-methyloxiranyl]carbonyl]butyl]-carbamic acid tert-butyl ester (6)
[0058] Using the same method as in Example 1, but changing the amount of diisopropylethylamine and pyridine sulfur trioxide to 0.12 mol, 0.08 mol of epoxidized product (6) was finally obtained, with a yield of 75.5%.
Embodiment 3
[0059] Example 3: [(1S,2S)-1-hydroxy-4-methyl-1-[[(2R)-2-methyloxiranyl]pent-2-yl] t-butyl carbamate ( 5) Synthesis
[0060]
[0061] Weigh 0.2mol [(3R,4S)-3-hydroxy-2,6-dimethyl-1-en-4-yl]carbamic acid tert-butyl ester (4), add 200ml of dichloromethane to dissolve, then add 0.04 mol vanadium acetylacetonate, under the protection of nitrogen, cool to 0℃ in an ice bath, slowly add tert-butanol peroxygen dropwise, double vigorous stirring overnight, TLC detects the disappearance of the raw materials, add appropriate amount of water and extract with dichloromethane, respectively with saturated thio After washing with sodium sulfate and saturated brine once, the organic phase was dried, concentrated and purified to obtain 0.12 mol of hydroxyl epoxidized product (5), with a yield of 60.8%.
[0062] 1 H NMR(600MHz, CDCl 3 )δ4.88(d,J=9.6Hz,1H),3.95-3.85(m,1H), 3.82(d,J=3.0Hz,1H), 2.97(d,J=4.8Hz,1H), 2.61( d,J=4.8Hz,1H),2.44(s,1H),1.69-1.57(m,1H),1.48-1.44(m,1H),1.43(s,9H),1.36(s,3H),1.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com