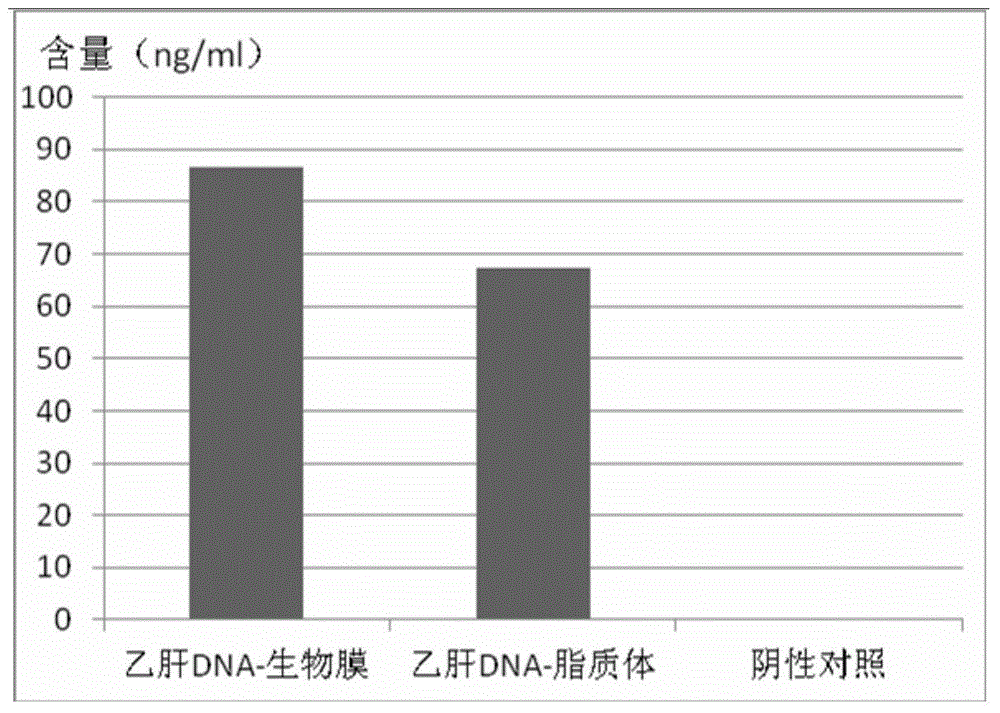
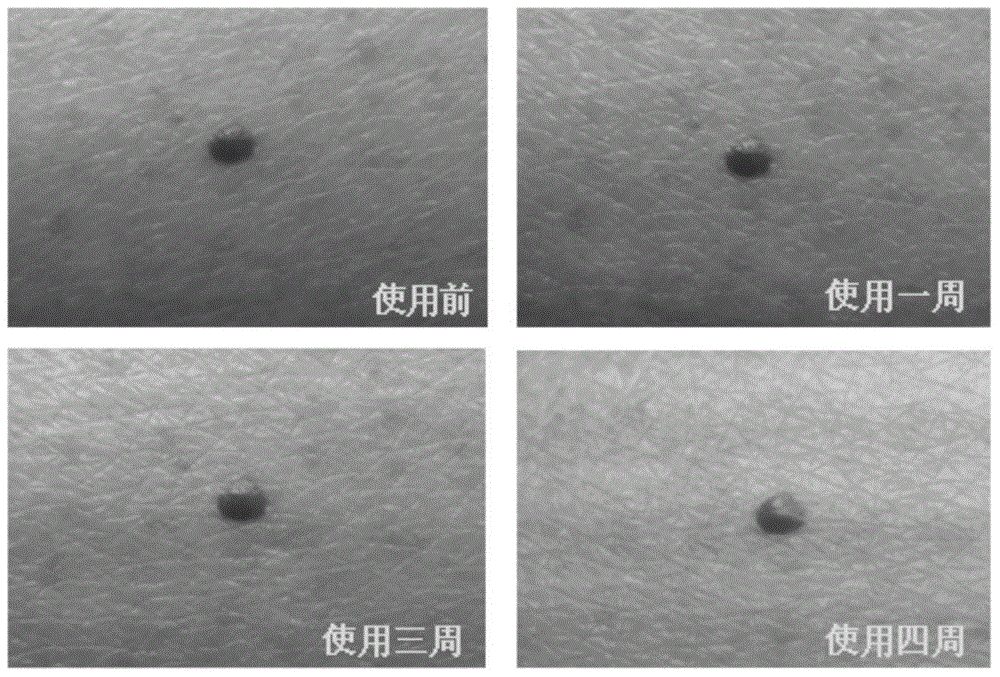
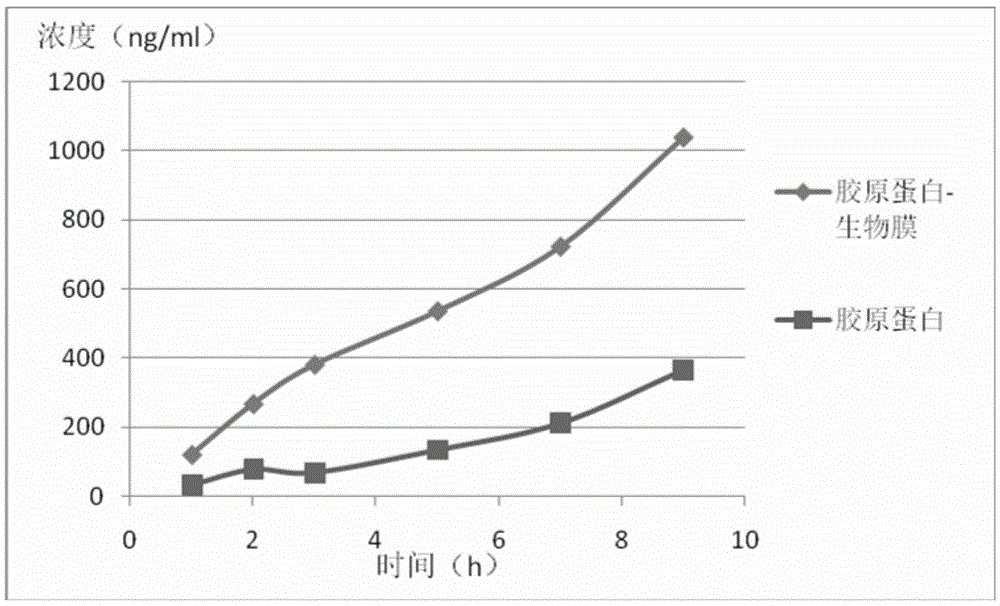
Application of biological membrane obtained by natural source and/or self-assembly technology, and closed structure or cellular compartment with biological membrane property as medicine carriers
A self-assembly technology, cell compartment technology, applied in the application field of closed structure and cell compartment as a medical carrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1 Extraction and purification of biofilm
[0076] The desired biofilm was extracted and purified by density gradient centrifugation. The specific process is as follows:
[0077] (1) New Zealand white rabbits that had been fasted for 18-24 hours were sacrificed to take the liver, and after removing the large blood vessels, the liver was cut into 2mm 3 The left and right small pieces were washed with normal saline until the tissue pieces turned white;
[0078] (2) Preparation of homogenate: 1mmol / L CaCl 2 , 50mmol / L HEPES (pH 7.4), 1mmol / L PMSF, 2μg / mL Aprotinin, 2μg / mL Antipain;
[0079] (3) Add liver tissue and homogenate to 8 times the volume of homogenate according to the mass:volume ratio, and homogenize with an electric homogenizer at 15,000 rpm in an ice-bath environment until the tissue is liquefied;
[0080] (4) Filter the homogenate through four layers of gauze, centrifuge the filtrate at 1,000×g, 10min, 4°C, and collect the precipitate;
[0081] ...
Embodiment 2
[0083] Example 2 Extraction and purification of biofilm
[0084] The desired biofilm was obtained by extracting and purifying by two-phase partition method. The specific process is as follows:
[0085] (1) Select plump and consistent normal corn seeds, soak them in 1% NaClO for 10 minutes, rinse them, and germinate them at 25°C in constant temperature and darkness for 72 hours;
[0086] (2) Take corn epicotyl, add 2 times the volume of extraction buffer (5mmol / L EDTA, 25mmol / L Tris, 0.25mmol / L sucrose, 1mmol / L MgSO 4 , 0.2% (W / V) BSA, 0.5% (W / V) PVP-10, 10% (W / V) glycerol, 15mmol / L β-mercaptoethanol, 1mmol / L PMSF, 1mmol / L DTT), ice bath grinding to liquefaction;
[0087] (3) Filter the grinding solution through four layers of gauze, centrifuge the filtrate at 12,000×g, 15min, 4°C, and take the supernatant;
[0088] (4) Centrifuge the supernatant at 80,000×g for 30 minutes at 4°C to collect the precipitate;
[0089] (5) Precipitate with suspension (25mmol / L Tris, 0.25mmo...
Embodiment 3
[0091] Example 3 Extraction and purification of biofilm
[0092] The desired biofilm was obtained by extracting and purifying by differential centrifugation. The specific process is as follows:
[0093] (1) Antarctic ice algae ( Chlamydomonas subcaudata ) is isolated and purified from Antarctic sea ice;
[0094] (2) Inoculate Antarctic ice algae into the medium at a ratio of 1:100 (21.2g NaCl, 3.6g NaSO per 10L medium 4 , 0.6g KCl, 0.3g NaHCO 3 , 0.1g KBr, 0.1g H 3 BO 3 , 0.1g NaF, 9.6g MgCl 2 ·6H 2 O, 1.0 g CaCl 2 , 0.1g SrCl 2 ·6H 2 O), cultured in a controlled light incubator. Cultivate for 14 days at -4°C, with a light intensity of 1300~1900lx, a light cycle of 12 light / 12 dark, and shaking 4~5 times a day;
[0095] (3) Centrifuge the ice algae culture solution at 4,000rpm, 20min, and 4°C to collect the ice algae sediment, and rinse it twice quickly with pre-cooled distilled water to remove extracellular viscous, surface salt and impurities in the culture so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com