Preparation method of lithium ion battery material lithium iron phosphate
A lithium ferrous phosphate and lithium-ion battery technology, applied in the field of lithium-ion batteries, can solve problems such as environmental hazards and no memory effect, and achieve the effects of easy desorption, good technical effects, and increased adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
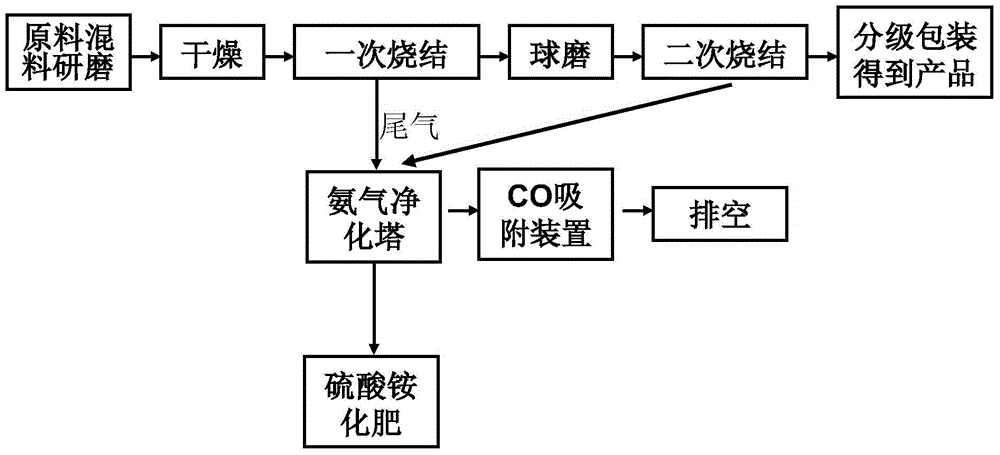
[0034]A preparation method of lithium iron phosphate lithium ion battery material, comprising the steps of:
[0035] Step (1), select raw materials for preparing lithium ferrous phosphate and prepare lithium ferrous phosphate product with high-temperature solid-state reaction method; The raw materials described in step (1) are iron source, phosphorus source, lithium source, specific iron source, phosphorus Source, lithium source are ferrous oxalate, ammonium dihydrogen phosphate, lithium carbonate respectively; Described high-temperature solid-state reaction method is: according to the molar ratio of iron source: phosphorus source: lithium source=1:1:1 mixing and grinding; After grinding and drying, transfer the material to a pre-calcining furnace for calcination at 600°C under a nitrogen atmosphere. The sintering time is 1 hour. After the first sintering, the material is ball-milled, and then the second sintering is carried out. The temperature of the second sintering is 650°C...
Embodiment 2
[0045] A preparation method of lithium iron phosphate lithium ion battery material, comprising the steps of:
[0046] Step (1), select raw materials for preparing lithium ferrous phosphate and prepare lithium ferrous phosphate product by high-temperature solid-state reaction method. The raw materials described in step (1) are iron source, phosphorus source and lithium source, and the specific iron source, phosphorus source and lithium source are respectively ferrous oxalate, ammonium dihydrogen phosphate and lithium carbonate. The high-temperature solid-phase reaction method is: mixing and grinding according to the molar ratio of iron source: phosphorus source: lithium source = 1:1:1. After grinding and drying, the material is transferred to a pre-calcining furnace for calcination under a nitrogen atmosphere at 700°C. The sintering time is 5 hours. After the first sintering, the material is ball-milled, and then the second sintering is carried out. The temperature of the secon...
Embodiment 3
[0055] A preparation method of lithium iron phosphate lithium ion battery material, comprising the steps of:
[0056] Step (1), select raw materials for preparing lithium ferrous phosphate and prepare lithium ferrous phosphate product by high-temperature solid-state reaction method. In step (1), iron source, phosphorus source, lithium source and carbon source are weighed, and specific iron source, phosphorus source, lithium source and carbon source are respectively ferrous oxalate, ammonium dihydrogen phosphate, lithium carbonate and glucose. The added amount is iron:phosphorus:lithium=1:1:1 (molar ratio), and the molar ratio of glucose to lithium source is 0.2:1. The high-temperature solid-phase reaction method is: mixing and grinding the raw materials according to the molar ratio. After grinding and drying, transfer the material to a pre-calcining furnace for calcination at 600°C under a nitrogen atmosphere. The sintering time is 1 hour. After the first sintering, the mater...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com