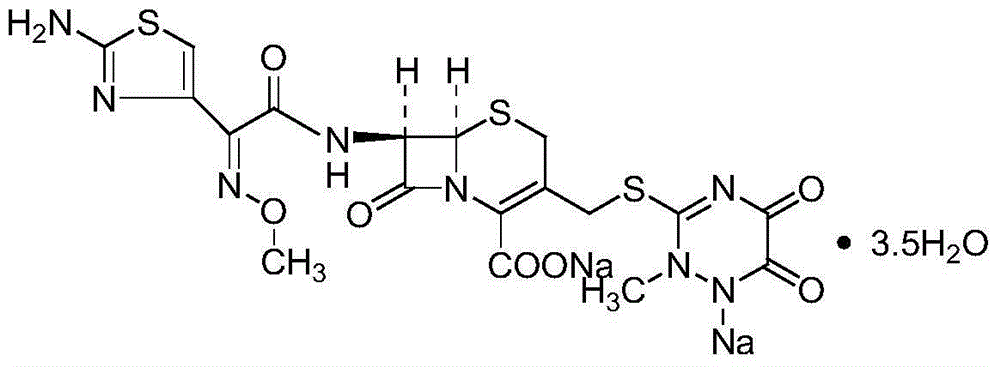
Preparation method of ceftriaxone sodium
A technology of ceftriaxone sodium and sodium acetate, which is applied in the direction of organic chemistry, can solve the problems of restricting product development prospects, multiple allergies, and high production costs, and achieve the effects of improving overall quality, extraction efficiency, and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Add 60ml of dichloromethane, 21ml of methanol, and 3ml of water into a 500ml reaction bottle, and cool down to -5~0°C.
[0031] (2) Add 7-ACT wet powder (approximately 18g in dry form) and 19.1g of AE active ester.
[0032] (3) 60min ~ 120min add 15ml triethylamine.
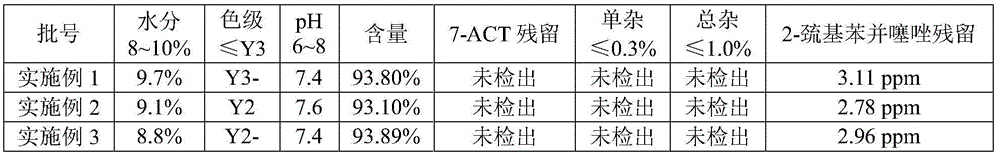
[0033] (4) Control the temperature at -3-3°C, time the reaction for 2 hours, and start sampling to measure the T salt residue. If the 7-ACT residue is ≤0.1%, it is qualified.
[0034] (5) After the 7-ACT residue is qualified, add (0.5g sodium metabisulfite+30ml water) solution, (7.2g sodium acetate+25ml water) solution, (1.05g sodium hydroxide+ 7ml of water), stirred and reacted for 30-60 minutes. Sodium transfer ends.
[0035] (6) Add an appropriate amount of dichloromethane to the reaction solution, and stir for 5 to 15 minutes to ensure that the phases can be separated.
[0036] (7) Stand still for 10 to 30 minutes, and separate the phases. One aqueous phase and one dichloromethane phase are ob...
Embodiment 2
[0044] (1) Add 60ml of dichloromethane, 21ml of methanol, and 3ml of water into a 500ml reaction bottle, and cool down to -5~0°C.
[0045] (2) Add 7-ACT wet powder (approximately 18g in dry form) and 19.1g of AE active ester.
[0046] (3) 60min ~ 120min add 15ml triethylamine.
[0047] (4) Control the temperature at -3-0°C, time the reaction for 2 hours, and start sampling to measure the T salt residue. If the 7-ACT residue is ≤0.1%, it is qualified.
[0048](5) After the 7-ACT residue is qualified, add (0.5g sodium metabisulfite+30ml water) solution, (7.25g sodium acetate+25ml water) solution, (1.25g sodium hydroxide+ 7ml of water), stirred and reacted for 30-60 minutes. Sodium transfer ends.
[0049] (6) Add an appropriate amount of dichloromethane to the reaction solution, and stir for 5 to 15 minutes to ensure that the phases can be separated.
[0050] (7) Stand still for 10 to 30 minutes, and separate the phases. One aqueous phase and one dichloromethane phase are ob...
Embodiment 3
[0058] (1) Add 60ml of dichloromethane, 21ml of methanol, and 3ml of water into a 500ml reaction bottle, and cool down to -5~0°C.
[0059] (2) Add 7-ACT wet powder (approximately 18g in dry form) and 19.1g of AE active ester.
[0060] (3) 60min ~ 120min add 15ml triethylamine.
[0061] (4) Control the temperature at 0-5°C, time the reaction for 2 hours, and start sampling to measure the T salt residue. If the 7-ACT residue is ≤0.1%, it is qualified.
[0062] (5) After the 7-ACT residue is qualified, add (0.5g sodium metabisulfite+30ml water) solution, (7.2g sodium acetate+25ml water) solution, (1.05g sodium hydroxide+ 7ml of water), stirred and reacted for 30-60 minutes. Sodium transfer ends.
[0063] (6) Add an appropriate amount of dichloromethane to the reaction solution, and stir for 5 to 15 minutes to ensure that the phases can be separated.
[0064] (7) Stand still for 10 to 30 minutes, and separate the phases. One aqueous phase and one dichloromethane phase are obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com