Method used for preparing epoxy compound via chlorohydrine intermediate cyclization catalyzed by supported solid base
A technology of alkali-catalyzed chlorohydrin and solid alkali catalyst, which is applied in the field of green chemistry, can solve the problems of ecological environment impact and cumbersome treatment, and achieve the effect of less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 5g of NaOH solid, add a small amount of water to configure NaOH aqueous solution. Weigh another 5gTiO 2 Put it in a beaker, add the above-mentioned prepared NaOH aqueous solution to it, and immerse in an equal volume at room temperature for 24 hours. Then dry it at 100~110°C to remove moisture, put it in a crucible, then bake it in a muffle furnace at 500°C for 5 hours, cool to room temperature and grind to get NaOH / TiO 2 Solid base catalyst with 50% NaOH loading. The alkali strength H was measured by the Hammett indicator method. - It is 15.0~18.4.
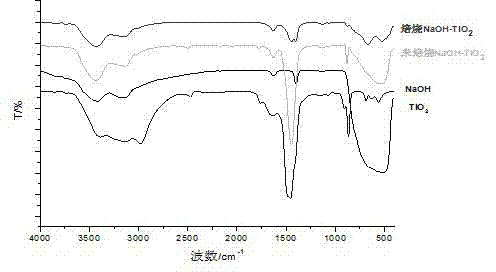
[0034] figure 2 For the prepared NaOH / TiO 2 The FT-IR spectrogram of solid base catalyst, by figure 2 It can be seen that NaOH / TiO 2 After solid alkali roasting, at 517cm -1 TiO 2 The absorption peak disappears at 670 cm -1 、876cm -1 There is an absorption peak at , so it can be judged that NaOH has been loaded on TiO 2 superior.
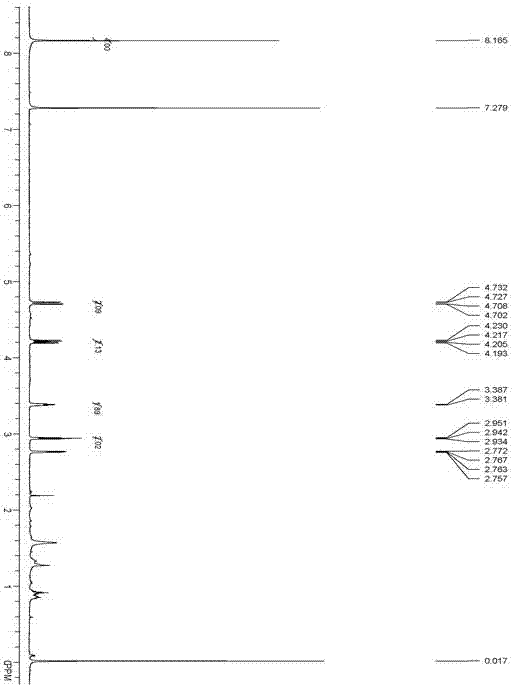
[0035] Add 1.66g of terephthalic acid, 14.8g of epichlorohydrin, and 0....
Embodiment 2~6
[0037] Except that the solid base catalyst type is different, all the other operations are the same as in Example 1, and the experimental results are shown in Table 1:
[0038] Table I:
[0039]
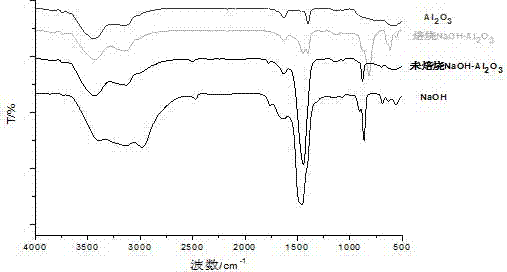
[0040] image 3 For the prepared NaOH / Al 2 o 3 The FT-IR spectrogram of solid base catalyst, by image 3 It can be seen that NaOH / Al 2 o 3 After solid alkali roasting, at 470 cm -1 O-Na-O absorption peak appears at 560cm -1 、617cm -1 Al-O absorption peak appears at 818cm -1 The O-O absorption peak appears at , so it can be judged that NaAlO is formed 2 ;1630 cm -1 、3000~3500cm -1 at OH - Absorption peak; 1450 cm -1 for CO 2 Absorption peak, and after roasting, this peak is obviously weakened.
Embodiment 7~10
[0042] According to the solid base preparation method described in Example 1, the loading capacity is respectively 10%, 20%, 30%, 40% solid base catalyst NaOH / TiO 2 , in the preparation process of diglycidyl terephthalate product, add above-mentioned different load capacity solid base catalyst respectively, all the other operations are identical with embodiment 1, experimental result sees table two:
[0043] Table II:
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com