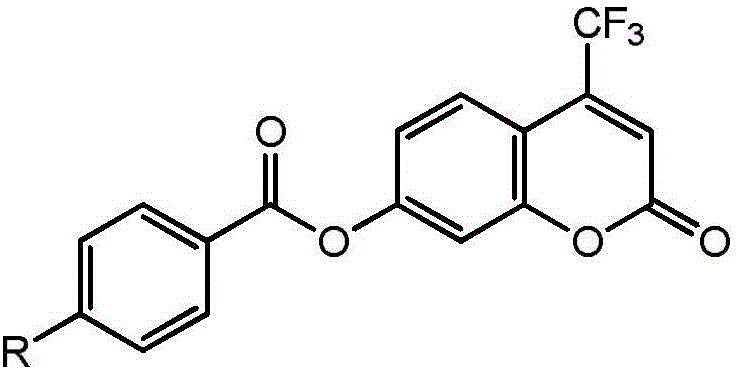
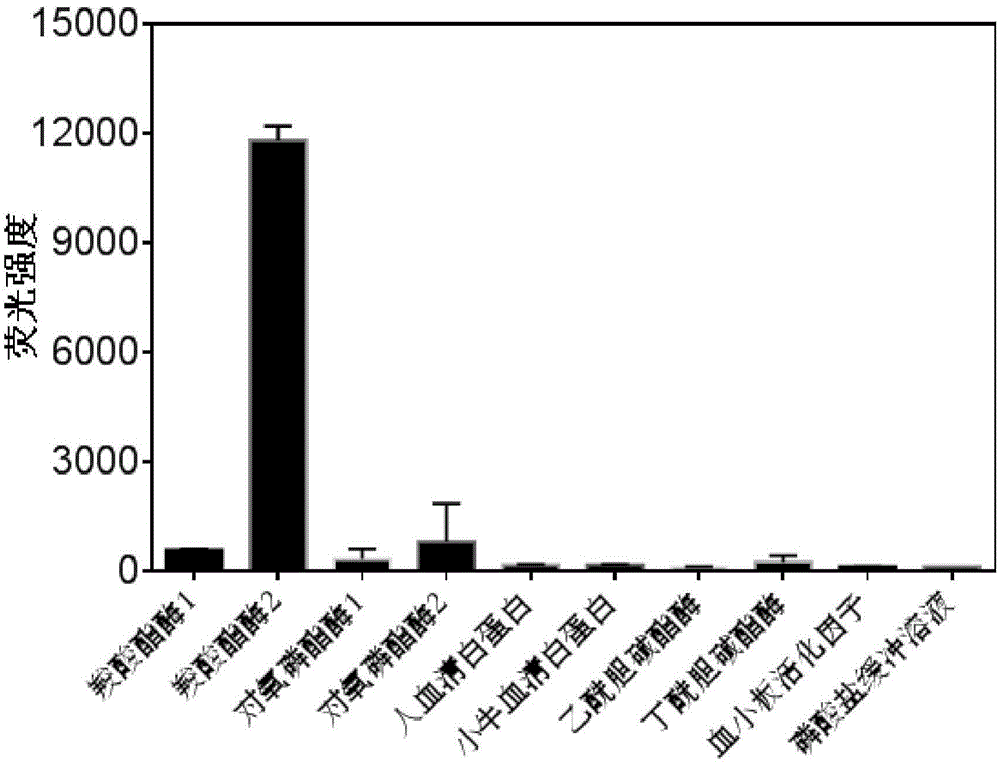
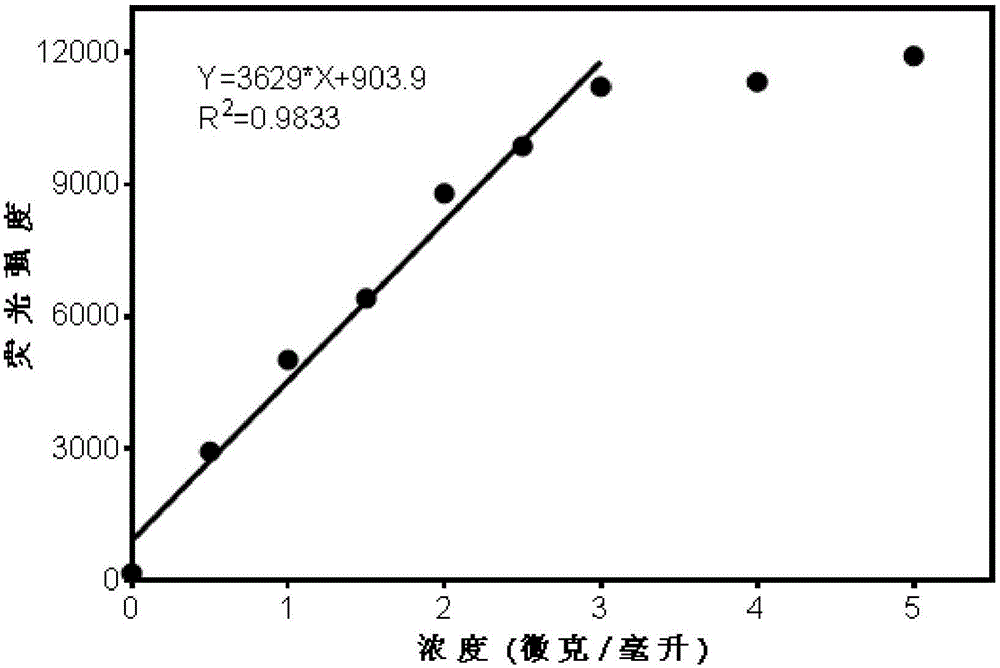
High-specificity fluorescent probe for human carboxylesterase hCE2 and application thereof
A high-specificity, fluorescent probe technology, applied in the field of medicine, to achieve high selectivity, fast and efficient evaluation of enzyme activity and inhibitory activity, and easy detection of metabolites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 The chemical synthesis of 4-trifluoromethyl-7-hydroxycoumarin 4-ethylbenzoyl ester
[0030] (1) To 10 mL of tetrahydrofuran solution containing 0.5 mmol of 4-trifluoromethyl-7-hydroxycoumarin and 0.625 mmol of triethylamine, slowly drop 0.6 mmol of p-ethylbenzoyl chloride (dissolved in 5 mL of in tetrahydrofuran), the temperature is controlled at 0°C;
[0031] (2) After stirring in an ice bath for 1 h, the solution was returned to room temperature and stirred overnight;
[0032] (3) The solvent was removed from the reaction solution under reduced pressure, and the residual solid was purified by silica gel chromatography, and eluted with ethyl acetate-n-hexane (1:3 v / v), to obtain 135 mg of pure white solid powder. 1 H NMR (400MHz, CDCl 3 )δ8.23–8.18(m,2H), 7.80(dt,J=8.8,1.8Hz,1H), 7.69(ddd,J=7.0,4.1,1.3Hz,1H), 7.59–7.49(m,2H) , 7.37 (d, J=2.3Hz, 1H), 7.29 (dd, J=8.8, 2.3Hz, 1H), 6.80 (s, 1H).
Embodiment 2
[0033] Example 2 The chemical synthesis of 4-trifluoromethyl-7-hydroxycoumarin 4-chlorobenzoyl ester
[0034] (1) To 10 mL of tetrahydrofuran solution containing 0.5 mmol of 4-trifluoromethyl-7-hydroxycoumarin and 0.625 mmol of triethylamine, slowly add 0.6 mmol of p-chlorobenzoyl chloride (dissolved in 5 mL of tetrahydrofuran In), the temperature is controlled at 0°C;
[0035] (2) After stirring in an ice bath for 1 h, the solution was returned to room temperature and stirred overnight;
[0036] (3) The solvent was removed from the reaction solution under reduced pressure, and the residual solid was purified by silica gel chromatography, and eluted with ethyl acetate-n-hexane (1:3 v / v), to obtain 132 mg of pure white solid powder.
Embodiment 3
[0037] Example 3 The chemical synthesis of 4-trifluoromethyl-7-hydroxycoumarin 4-methylbenzoyl ester
[0038] (1) To 10 mL of tetrahydrofuran solution containing 0.5 mmol of 4-trifluoromethyl-7-hydroxycoumarin and 0.625 mmol of triethylamine, slowly drop 0.6 mmol of p-toluyl chloride (dissolved in 5 mL of in tetrahydrofuran), the temperature is controlled at 0°C;
[0039] (2) After stirring in an ice bath for 1 h, the solution was returned to room temperature and stirred overnight;
[0040] (3) The solvent was removed from the reaction solution under reduced pressure, and the residual solid was purified by silica gel chromatography, and eluted with ethyl acetate-n-hexane (1:3 v / v), to obtain 131 mg of pure white solid powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com