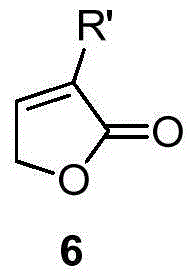
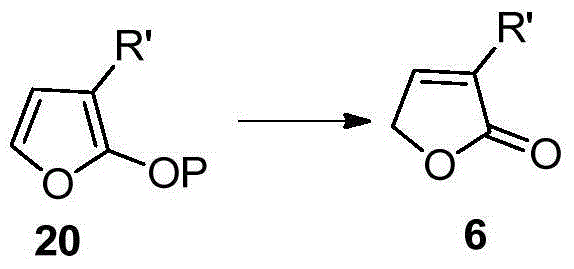
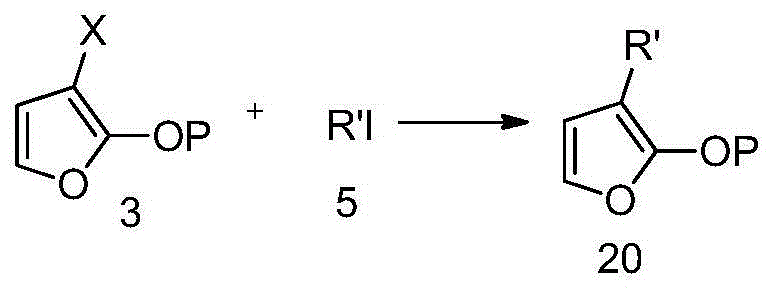
Isotopically labeled methyl furanone, intermediate and preparation method of isotopically labeled methyl furanone
An isotope labeling, methyl furanone technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of harsh synthesis conditions, poor stability, low deuterium abundance of strigolactone, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1 Synthesis of 3-bromofuran-2[5H]-one (compound 2) (according to literature Boukouvalas, J.; Loach, R.P. General, Regiodefined Access to α-Substituted Butenolidesthrough Metal-Halogen Exchange of 3-Bromo-2- Silyloxyfurans. Efficient Synthesis of an Anti-Inflammatory Gorgonian Lipid. J. Org. Chem. 2008, 73, 8109–8112. Reported method synthesis)
[0100]
[0101] Build the experimental device: 100mL three-neck flask, reflux condenser, constant pressure dropping funnel and thermometer, under nitrogen protection, add 3.13g (37.3mmol) furanone and 40mL anhydrous ether, and use an ice bath to lower the system temperature to 0°C. Then, 2.2 mL (43.4 mmol) of bromine and 10 mL of anhydrous ether were added to the dropping funnel, and the above solution was slowly added dropwise to the reaction system, and the dropwise addition was completed in 25 minutes. After dripping, reflux (about 35°C) to react for 4h. Then nitrogen gas was blown into the system for 1 hour to re...
Embodiment 2
[0102]Example 2 Synthesis of 2-triisopropylsilyloxy-3-bromofuran-2[5H]-one (compound 3) (according to literature Boukouvalas, J.; Loach, R.P.General, Regiodefined Access to α-Substituted Butenolides through Metal-Halogen Exchange of 3-Bromo-2-Silyloxyfurans. Efficient Synthesis of an Anti-Inflammatory Gorgonian Lipid. J. Org. Chem. 2008, 73, 8109–8112. Reported method synthesis)
[0103]
[0104] Add 0.503g (3.1mmol) 3-bromofuran-2[5H]-one and 20mL of dried dichloromethane into the Schlenk tube that has been replaced with nitrogen, cool the temperature to below 0°C in an ice-salt bath, and measure 0.56mL ( 4mmol) triethylamine, added dropwise in the reaction tube and finished dripping in 10 minutes, the reaction solution immediately turned dark brown from light yellow, added dropwise 1.242g (4mmol) triisopropylmethyl trifluoromethanesulfonate, and reacted Liquor turns orange-red immediately, then gradually turns into wine red, keeps stirring in ice-salt bath for 30min after...
Embodiment 3
[0105] Example 3 CD 3 -Synthesis of iodomethane (Compound 5A)
[0106]
[0107] Add 5mL deuterated methanol, 5mL water and 50mL hydroiodic acid (mass percentage is 55.0%-58.0%) in reaction flask, and described mass percentage refers to the percentage that the quality of hydrogen iodide accounts for hydroiodic acid gross mass; Phosphoric acid stabilizer mass percentage is 1.5%, and described mass percentage refers to the mass percentage of hypophosphorous acid stabilizer accounted for hydroiodic acid reagent gross mass), stirs, is heated to 40 ℃ of reaction 2h, is heated to 50 ℃ of reaction 2h, drops After reaching room temperature (about 20°C), replace it with a distillation apparatus, collect fractions at 40°C to 45°C under normal pressure (1 atm), and distill at a rate of 0.1mL / s to obtain 13.25g of a colorless liquid with a yield of 83.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com