Recombinant human brain natriuretic peptide injection and preparation method thereof
A technology for human brain natriuretic peptides and injections, which is applied in pharmaceutical formulations, drug combinations, peptide/protein components, etc. It can solve the problems of high requirements for product dosage accuracy, cumbersomeness, secondary pollution, etc., and achieve good stability Sex and activity, easy to use, accurate effect of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] 1. Dose selection:
[0027] This product adopts the combination of intravenous push injection and continuous intravenous infusion. Firstly, after the intravenous pulse of 1.5 μg / kg, it is continuously intravenously infused at a rate of 0.0075 μg / kg / min for 24 hours; loading dose: 1.5-2 μg / kg, maintenance dose rate: 0.0075-0.01 μg / kg / min. This patent is calculated based on the average body weight of domestic patients and the method of administration, and finally selects 500 μg / ml (dose) with reference to the specifications of the marketed products.
[0028] 2. Buffer system screening:
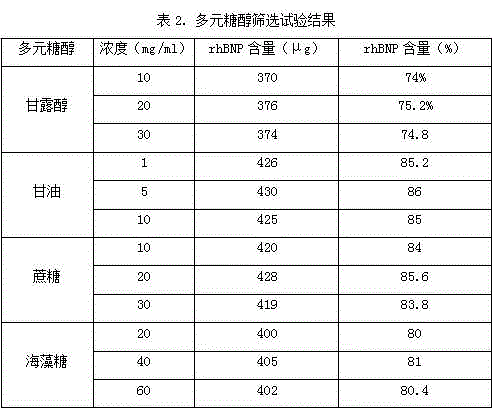
[0029] To investigate the effects of different buffers, different pH values and different buffer concentrations on the stability of recombinant human brain natriuretic peptide, 500 μg recombinant human brain natriuretic peptide was placed in 1 ml of different types of buffers, and the buffer systems were respectively Phosphate buffer system, acetate buffer system and citrate buffer sy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| buffer concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com