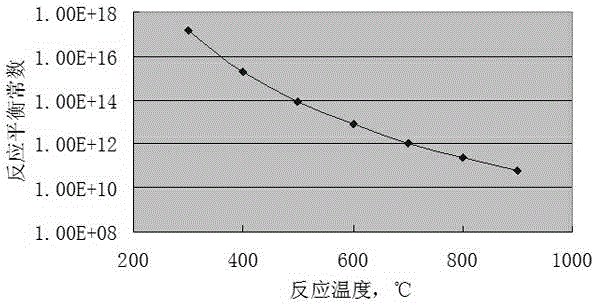
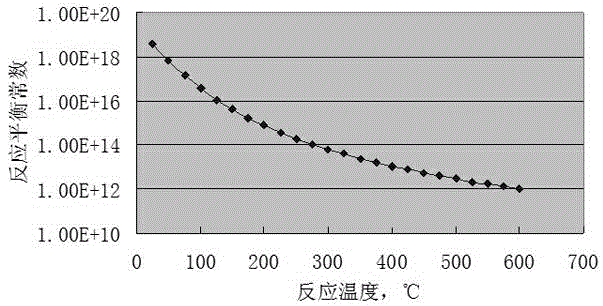
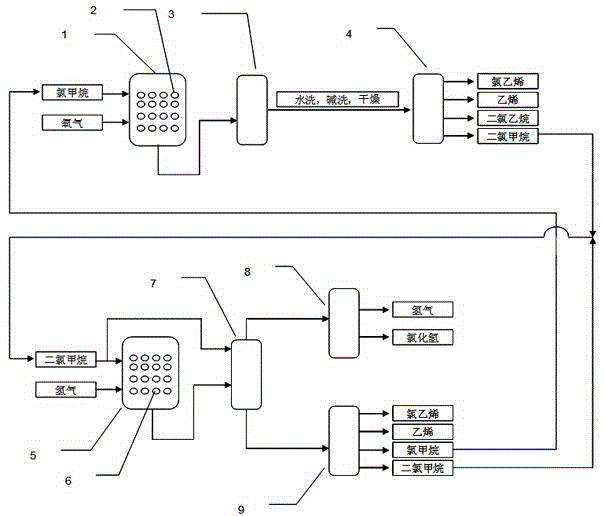
Method for preparing vinyl chloride monomer by using methane chloride
A technology of vinyl chloride monomer and methane chloride, which is applied in chemical instruments and methods, preparation of halogenated hydrocarbons, chemical/physical processes, etc., and can solve problems such as low selectivity of vinyl chloride, many types of products, and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1: Preparation of vinyl chloride monomer by oxidative coupling reaction of methyl chloride alone
[0095] The catalyst is obtained by dissolving a certain mass of precursors of barium, tungsten and potassium in water, adding silicon-aluminum molecular sieves, stirring evenly, drying and roasting. Mix methyl chloride and oxygen at a molar ratio of 1:1, pass it into a reactor equipped with a catalyst, the reaction temperature is 600°C, the reaction pressure is normal pressure, the reaction effluent is cooled to room temperature, washed with water and alkali to remove hydrogen chloride, condensed Remove water, pressurize, cool and separate to obtain liquid vinyl chloride. The conversion rate of methyl chloride is about 50.0%, the selectivity of vinyl chloride is over 70.0%, and the selectivity of dichloromethane is 15.0%. Calculation shows that vinyl chloride yield is 35.0%.
Embodiment 2
[0096] Embodiment 2: Using the oxidative coupling reaction method of methyl chloride alone to prepare vinyl chloride monomer
[0097] The catalyst is prepared by dissolving a certain mass of precursors of cobalt, molybdenum and cerium in water, adding silica-alumina molecular sieves, mixing evenly, drying and roasting. Mix methyl chloride and oxygen at a molar ratio of 1:1, pass it into a reactor equipped with a catalyst, the reaction temperature is 600°C, the reaction pressure is normal pressure, the reaction effluent is cooled to room temperature, washed with water and alkali to remove hydrogen chloride, condensed Remove water, pressurize, cool and separate to obtain liquid vinyl chloride. The conversion rate of methyl chloride is about 60.0%, the selectivity of vinyl chloride is over 70.0%, and the selectivity of dichloromethane is 23.0%. Calculation shows that vinyl chloride yield is 42.0%.
Embodiment 3
[0098] Embodiment 3: Use dichloromethane hydrocoupling reaction method alone to prepare vinyl chloride monomer
[0099] Precursor compounds of cobalt, molybdenum, iron and potassium are made into a solution according to a certain ratio, and alumina is used as a carrier, impregnated by an incipient wetness impregnation method, and then dried and roasted to obtain a catalyst. Hydrogen and dichloromethane (gasified by heating) were mixed according to a molar ratio of 3:1, passed into the reactor filled with the above catalyst, and reacted at 450 °C and 1 atm. After the reaction effluent is treated by the subsequent cooling and separation system, products such as vinyl chloride, ethylene and methyl chloride are obtained, and the selectivities are 42.0%, 17.0%, and 25.0%, respectively. The methylene chloride conversion was about 46.0%. Calculation shows that vinyl chloride yield is 19.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com