A liquid formulation of long-acting insulin conjugate
A technology for long-acting insulin and liquid preparations, which can be used in medical preparations without active ingredients, medical preparations containing active ingredients, and drug combinations, etc. It can solve problems such as affecting protein stability and achieve the effect of increasing the duration of the body.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1: Identification of Factors Determining Stability of Long-Acting Insulin Conjugate Liquid Formulations
[0099] Long-acting insulin conjugates have been developed that have an increased half-life in blood without causing low blood sugar levels in the body. Insulin conjugates - in which the immunoglobulin Fc region, non-peptidyl polymer, and insulin are site-specifically bound by covalent bonding - have increased half-life in blood and can significantly reduce hypoglycemia levels danger.
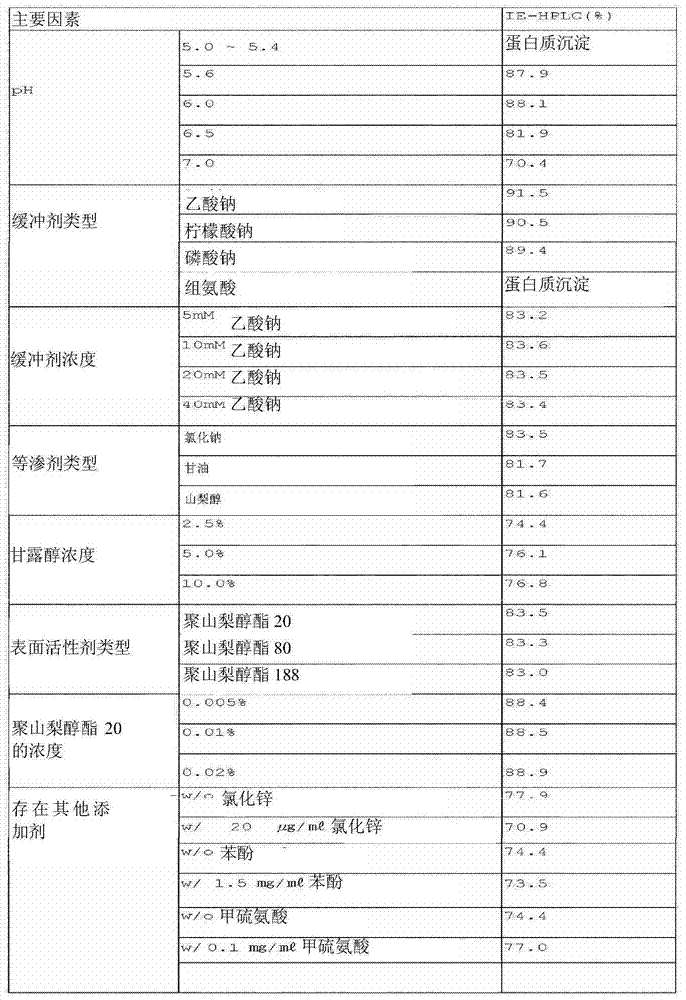
[0100] To confirm the stability of the liquid formulation of the long-acting insulin conjugate, the formulation was prepared with the composition of Table 1 and stored at 40°C for 2 weeks, and its stability was analyzed by ion exchange chromatography (IE-HPLC).
[0101] At this time, the main factors that were compared to determine its effect on the stability of the conjugate were pH, type and concentration of buffer, type of isotonicity agent, concentration of sugar alcoho...
Embodiment 2
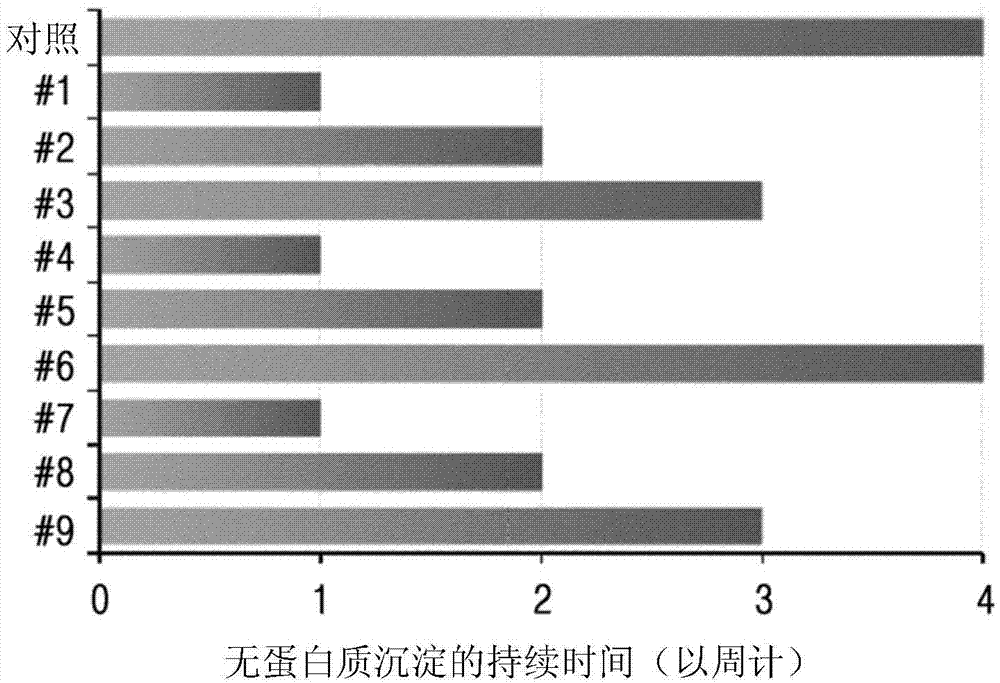
[0108] Example 2: Evaluation of long-acting insulin conjugate stability according to isotonicity agent and surfactant concentration
[0109] Based on the liquid formulation confirmed in Example 1 (10 mM sodium acetate at pH 6.0, 10 ㎎ / Sodium chloride, 10% (w / v) mannitol, 0.02% (w / v) polysorbate 20), depending on the concentration of isotonicity and surfactant, the stability of the long-acting insulin conjugates was tested. At this point, the concentrations of isotonicity agent and surfactant are set within the maximum acceptable range recommended by commercial formulations and licensing agencies.
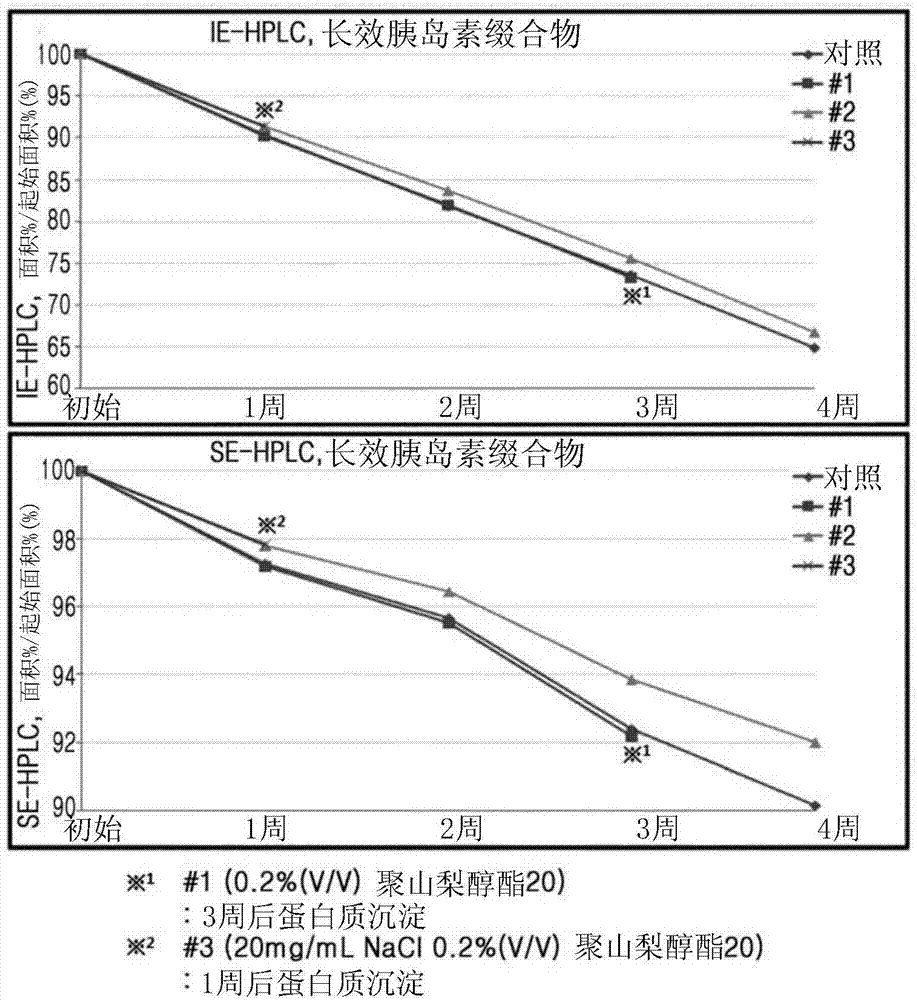
[0110] Liquid formulations of long-acting insulin conjugates were prepared with the composition of Table 3 and stored at 40°C for 4 weeks. Then, stability was checked by IE-HPLC and size exclusion chromatography (SE-HPLC).
[0111] The IE-HPLC (%) and SE-HPLC (%) results in Table 4 represent "area % / initial area %" values, indicating the remaining purity of the long-acting insu...
Embodiment 3
[0118] Example 3: Stability evaluation of long-acting insulin conjugates according to sugar alcohol type
[0119] Examples of sugar alcohols that can be added to the formulation to enhance the storage stability of the long-acting insulin conjugate include monosaccharides such as mannose, glucose, fucose and xylose; and polysaccharides such as lactose, maltose, sucrose, raffinose , and dextran. Among them, the effect of sucrose on the stability of long-acting insulin conjugates was tested, since sucrose was confirmed to have the effect of reducing deamidation (J. of Pharmaceutical Sciences, Vol. 94, 2005). At this point, the sucrose concentration is within the maximum acceptable range recommended by commercial formulations and licensing agencies.
[0120] Liquid formulations of long-acting insulin conjugates were prepared with the composition of Table 5 and stored at 40°C for 4 weeks, and their stability was checked by stability testing using IE-HPLC and SE-HPLC. The IE-HPL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com