Excipient-containing lyophilized paclitaxel powder preparation and preparation method thereof
A technology of paclitaxel and freeze-dried powder, which is applied in the direction of freeze-dried delivery, powder delivery, antineoplastic drugs, etc. It can solve the problems of large particle size and complicated process, and achieve the effect of simple formula, good solubility and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment (1-7
[0037] A paclitaxel freeze-dried powder preparation containing excipients is prepared according to the following process steps.
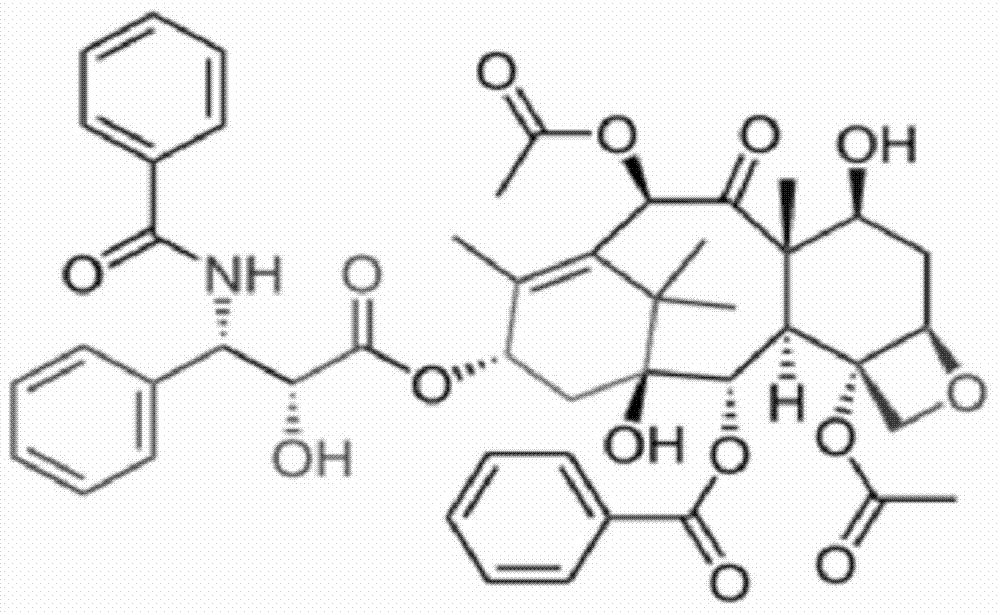
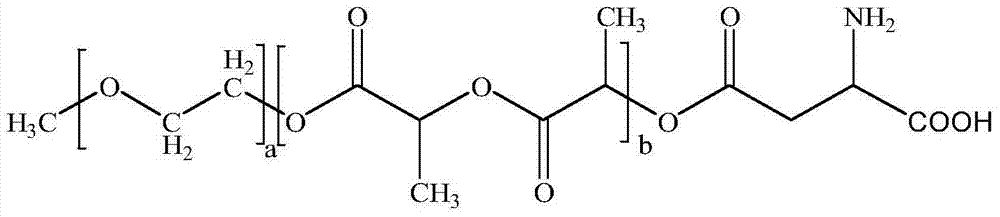
[0038] ① Weigh raw materials according to different feeding ratios (paclitaxel: mPEG-PLA-aspartic acid). Among them, the raw material paclitaxel (CAS33069-62-4) is produced by Xi'an Ruilin Biotechnology Co., Ltd., with a purity greater than 95%, and the raw material mPEG-PLA-aspartic acid is prepared by the inventor according to the patent No. PCT-CN-2013000453 Self-preparation process;
[0039] ②Put the above-mentioned raw materials into a container, and add organic solvents such as ethanol or acetonitrile at a temperature of 15-45°C until they are completely dissolved. The dissolving process can adopt means such as stirring or shaking.
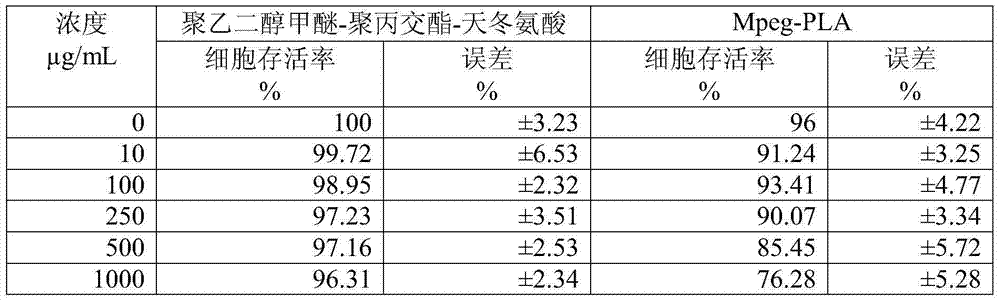
[0040] ③ Rotate the above solution at 30-50°C for 2 hours until the organic solvent evaporates to dryness. Then vacuum drying at 10-40° C. for > 12 hours to remove residual organic solvents to obtain a paclitax...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com