Cu-Eu red fluorescent and magnetic microporous structure complex and preparation method thereof
A technology of red fluorescence and microporous structure, which is applied in the field of luminescent materials and magnetic materials, to achieve the effects of improving luminous efficiency, improving light conversion efficiency, and novel geometric topological structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
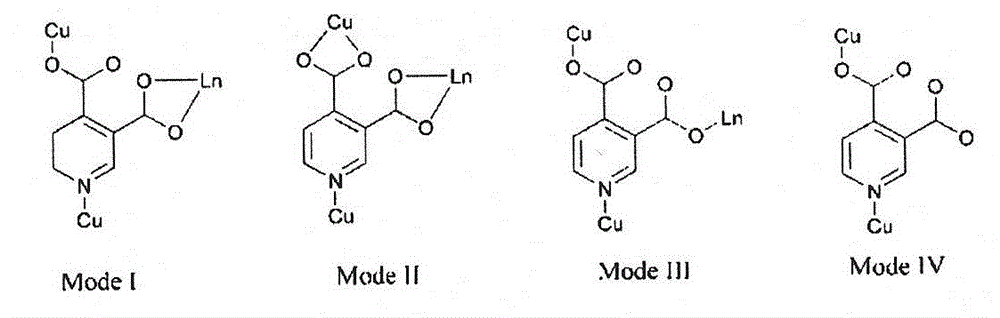
[0045] Synthesis of red fluorescent single crystals with metal-organic framework microporous structure. Add CuCl in a beaker at a molar ratio of 3:2:6:12 2 , EuCl 3 , 3,4 dipicolinic acid and triethylamine. Reflux for 2 hours and filter to obtain a light blue solution. After standing for three days, sky blue hexagonal microcrystals appeared, with a yield of 45%. Pick a blue-green crystal of 0.2mm×0.18mm×0.12mm, use Xcalibur single crystal X-ray diffractometer, use Mo Kα ray (λ=0.71073nm) monochromated by graphite, and collect diffraction by φ-ω scanning data. Diffraction intensities were Lp corrected. The empirical absorption correction was carried out. The single crystal structure was solved by the direct method, and all non-hydrogen atoms were found by the difference Fourier synthesis method, and the coordinates of all non-hydrogen atoms were corrected by the least square method. The molecular structure of the complex is shown in figure 2 shown. Depend on figure 2 ...
Embodiment 2
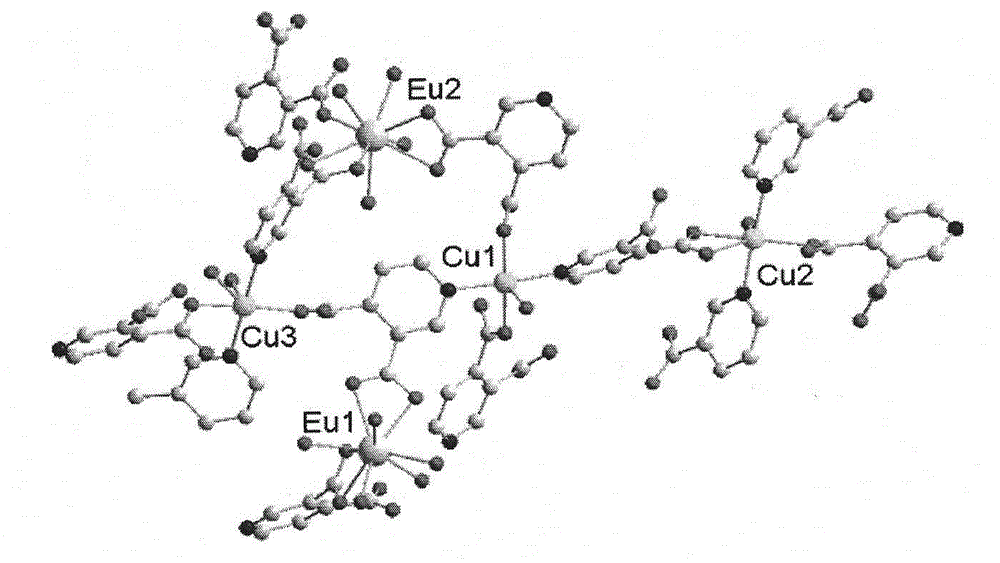
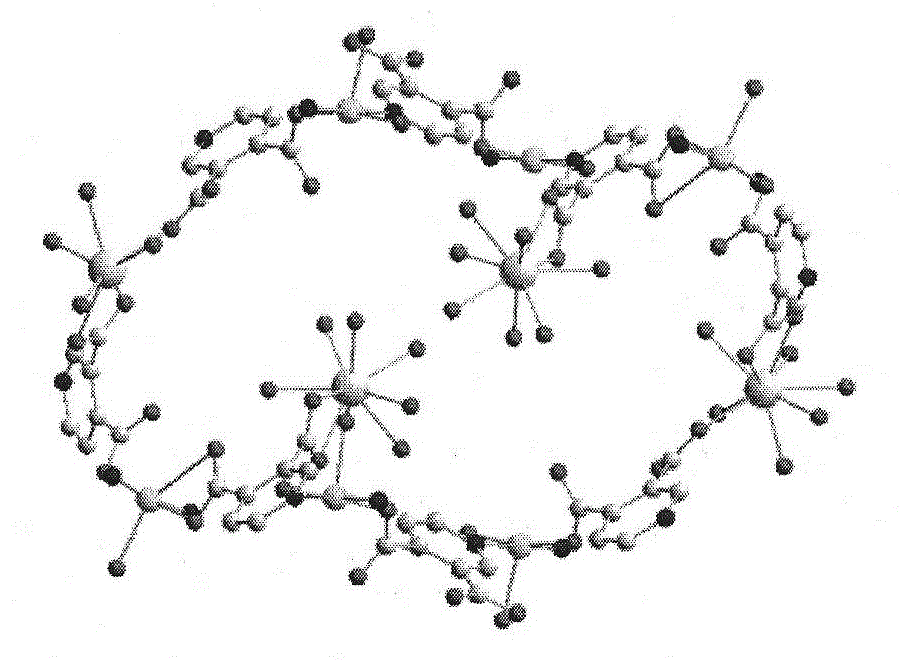
[0047] Add 3,4 dipicolinic acid and triethylamine in ethanol to prepare solution A in a molar ratio of 6:12, and mix Cu(NO 3 ) 2 , Eu(NO3) 3 Dissolve in water to prepare solution B, mix solution A and solution B, stir for about 8 hours, filter to obtain a light blue solution, after standing for three days, sky blue hexagonal microcrystals appear. Further analysis of the structure of complex 1 as image 3 , it can be seen that the complex has a three-dimensional hole structure, and the hole radius is about 0.6nm. Table 1 is a partial Eu...Cu and Cu...Cu spacing table. It can be seen from Figure 4 that each copper ion connects four 3,4-pyridinedicarboxylic acid molecules, and each 3,4-pyridinedicarboxylic acid molecule bridges two copper ions to form an extended irregular two-dimensional plane , Europium ions are located between these two-dimensional planes, linking the copper ion coordination units to form a three-dimensional framework structure. Among them, Eu1 connects 4...
Embodiment 3
[0052] Add 3,4 dipicolinic acid and triethylamine in ethanol at a molar ratio of 6:12 to prepare solution A, and dissolve CuSO at a molar ratio of 3:2 4 , Eu(CH 3 COO) 3 Dissolve in water to prepare solution B, mix solution A and solution B, stir for about 10 hours, filter, and stand still to obtain a single crystal. Drying in the air gave blue powder with a yield of 46%. Compound 1 was further tested by polycrystalline powder diffraction as shown in Figure 5 , according to the simulation value of the single crystal structure and the experimental test value are in good agreement, which proves that the prepared powder has a high purity. In order to further test the stability of the complex, a thermogravimetric test was performed at room temperature -750°C. Depend on Image 6 It can be seen that the complex begins to lose crystal water in the range of 65-180°C, and the weight loss rate is about 24%, which is more consistent with the theoretical value. The complex began to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com