A High Performance Liquid Chromatography Separation Column Suitable for Chiral Separation of Amino Acids
A high-performance liquid chromatography and chiral separation technology, which is applied in the field of high-performance liquid chromatography separation columns for chiral separation of amino acids, can solve problems such as harsh temperature conditions, no separation effect, and no solution, and achieve separation High speed, low cost of column making, high resolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
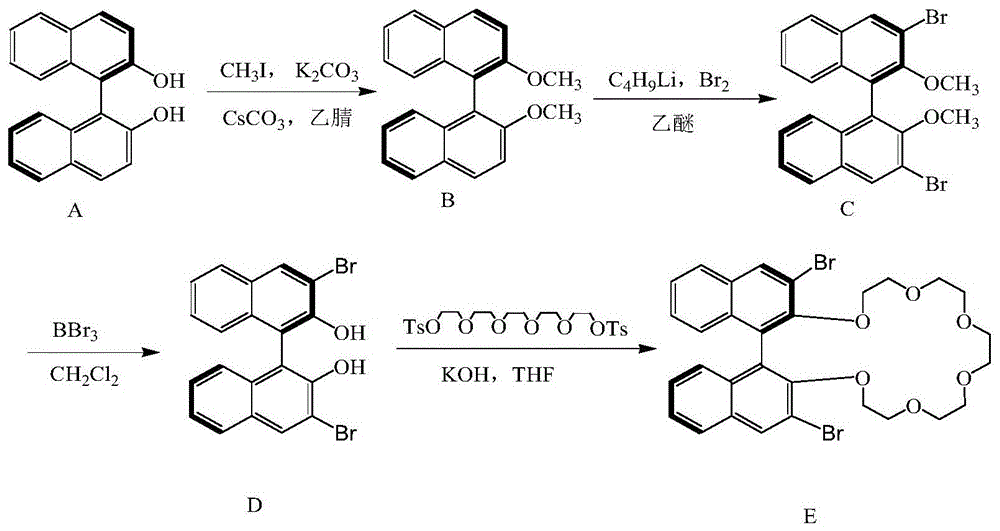
[0018] The method for synthesizing R-(3,3'-dibromo-1,1'-dinaphthyl)-20-crown-6 (such as figure 1 Shown): R-binaphthol 10g is added in 180mL acetonitrile, adds 20g K 2 CO 3 and 0.5g CsCO 3 As a catalyst, stir under magnetic reflux at 70°C, slowly add 6.52 mL of methyl iodide dropwise during the reflux process, and react for 4 hours. The mixture was extracted with dichloromethane, the organic layer was dried and purified by silica gel column [eluent V (ethyl acetate): V (petroleum ether) = 1:5], and the eluent was removed under reduced pressure to obtain 10.54 g of white crystal B; Under the protection of nitrogen, 37.6mL of normal C 4 h 9 Li (1.6mol / L) was added to a solution of tetramethyldiethylamine (6.1mL) in 200mL of ether, stirred at 25°C for 15min, and 8.0g of product B was added and stirred for 3h. Then the reactant was cooled to -75°C, and Br was added within 10 min. 2 (3.9mL) in pentane (20mL) solution, then the reaction was raised to room temperature and stirre...
Embodiment 2
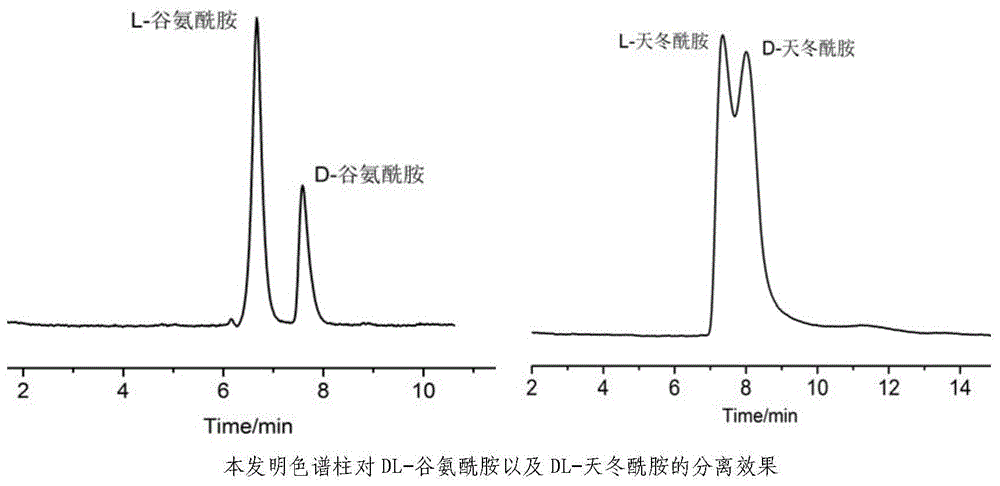
[0023] With the chromatographic column obtained in Example 1, the detection wavelength of the ultraviolet detector is 210nm, the mobile phase is pH=2, and the perchloric acid solution that is ultrasonically degassed is filtered through a 0.45 μm filter membrane, and the flow rate is 0.5mL min -1 , under the condition that the column temperature of the liquid phase column is 25°C, test the liquid chromatography chiral resolution effect on DL-glutamine and DL-asparagine, see image 3 .
[0024] Depend on image 3 It can be seen that the chromatographic column obtained in Example 1 can achieve baseline separation for DL-glutamine and partial separation for DL-asparagine, indicating that it also has a certain separation effect for non-protein α-chiral primary amines.
Embodiment 3
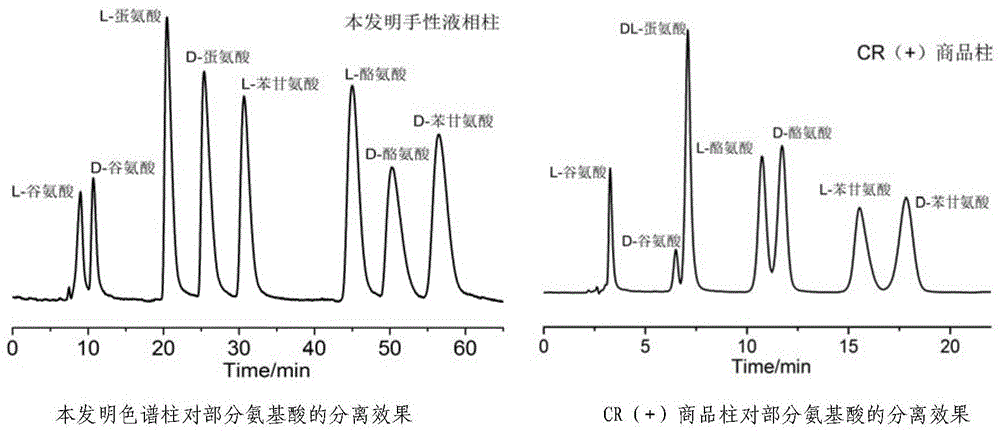
[0026] With the chromatographic column of the present invention a (Embodiment 1 gained) and commodity CR (+) column b The chiral column detects 21 kinds of α-amino acid enantiomers (the first 19 are amino acids that make up proteins, and the latter two are other chiral primary amines). The detection wavelength of the ultraviolet detector is 210nm, and the mobile phase is pH=1. Filter the ultrasonically degassed perchloric acid solution with a μm filter membrane at a flow rate of 0.5mL min -1 , the chromatographic chiral resolution effect under the condition that the column temperature of the liquid phase column is 25°C. retention factor k 1 =(t 1 -t 0 ) / t 0 ,k 2 =(t 2 -t 0 ) / t 0 ;Separation factor α=k 2 / k 1 ; Resolution Rs=1.18(t 2 -t 1 ) / (W 1 / 2 (1)+W 1 / 2 (2)); where t 1 , t 2 Respectively, the retention time of the first elution peak and the second elution peak of the sample; W 1 / 2 (1), W 1 / 2 (2) are respectively the half-peak widths of two enantiomer chro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com