Method for producing inactivated vaccine of H9N2 subtype of avian influenza virus and product of inactivated vaccine
A production method and technology of inactivated vaccines, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, antibody medical components, etc., can solve problems such as the production of H9N2 subtype avian influenza inactivated vaccines, and meet the requirements of immunization production, Effect of reducing contamination risk and saving cultivation space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]Example 1 Isolation and Identification of H9N2 Subtype Avian Influenza Virus NJ13-8 Strain
[0037] 1. Experimental method
[0038] 1.1 Isolation of virus
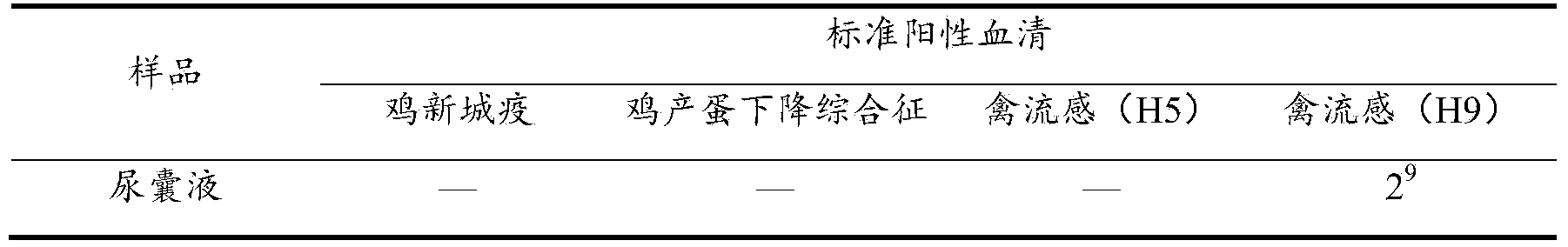
[0039] In 2013, the lungs and livers were collected from a chicken farm in Nanjing, Jiangsu Province, and sent for inspection. The lungs and livers were cut into pieces, added with sterilized saline at a ratio of 1:5, ground in a sterile mortar, frozen and thawed three times repeatedly, and subjected to 3000r / min centrifuged for 15min, and the supernatant was taken for later use.
[0040] The supernatant of the above-mentioned disease material was sterilized through a 0.22 μm disposable filter, and inoculated with 10-day-old SPF chicken embryos through the allantoic cavity, with 0.1 ml for each chicken embryo, and a total of 5 chicken embryos were inoculated. Incubate at 37°C; discard dead embryos within 24 hours, aseptically collect the allantoic fluid of chicken embryos that died within 24-120 hours, and store a...
Embodiment 2
[0067] Example 2 Pathogenicity assay of isolated virus NJ13-8 strain and its proliferation in MDCK cells
[0068] 1. Experimental method
[0069] 1.1 Pathogenicity determination of isolated virus NJ13-8 strain
[0070] Embryo median infectious dose (EID) 50 ), intracerebral pathogenicity index (ICPI) and intravenous inoculation index (IVPI) test to detect the pathogenicity of the virus NJ13-8 strain that embodiment 1 isolates.
[0071] 1.1.1 Virus EIDs 50 determination
[0072] Make the isolated virus with sterilized saline for 10 -6 ~10 -9 Dilution, and set up a blank control group, inoculate 5 10-day-old SPF chicken embryos at each dilution, discard 24h dead embryos, freeze the chicken embryos overnight at 4°C 120h after inoculation, harvest chicken embryo allantoic fluid, and measure its HA efficacy Valence, and refer to the Reed-Muench method to calculate the virus EID 50 .
[0073] 1.1.2 Determination of intracerebral pathogenicity index (ICPI)
[0074] Take 10 1...
Embodiment 3
[0099] Example 3 Preparation of Inactivated Vaccine Using Cell Factory to Cultivate H9N2 Subtype Avian Influenza Virus
[0100] 1. Experimental method
[0101] 1.1 Cell culture
[0102] First, resuscitate MDCK cells into T75 cell flasks, grow for about 48 hours, and pass passage according to the ratio of 1:4; then transfer the cells in 6 T75 cell flasks full of cells to a 10L spinner bottle for culture; about 48 hours Subculture the cells at a ratio of 1:3; the medium used in this process is DMEM, the serum is fetal bovine serum, and the usage amount is 10%.
[0103] The cells obtained in the spinner bottle were washed twice with PBS, and then 125 ml of 0.25% trypsin-EDTA solution was added for digestion, and the cells in the spinner bottle were harvested.
[0104] After the harvested cells were counted, the MDCK cells were cultured in the Thermo Scientific Nunc cell factory, and the medium used was DMEM medium. Samples were taken at 12h, 24h, and 48h after cell inoculation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com