Application of hyperbranched polyglycidyl ether modified nano-magnetic microspheres in chemiluminescence immunoassay
A chemiluminescence immunoassay, glycidyl ether technology, applied in analytical materials, scientific instruments, measuring devices, etc., can solve the problems of low coupling efficiency, inability to break through the maximum theoretical density of single-layer surface, and limiting the amount of antibody coupling, etc. Achieve the effect of reducing non-specific adsorption, optimizing the minimum detection limit, and broadening the detection range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
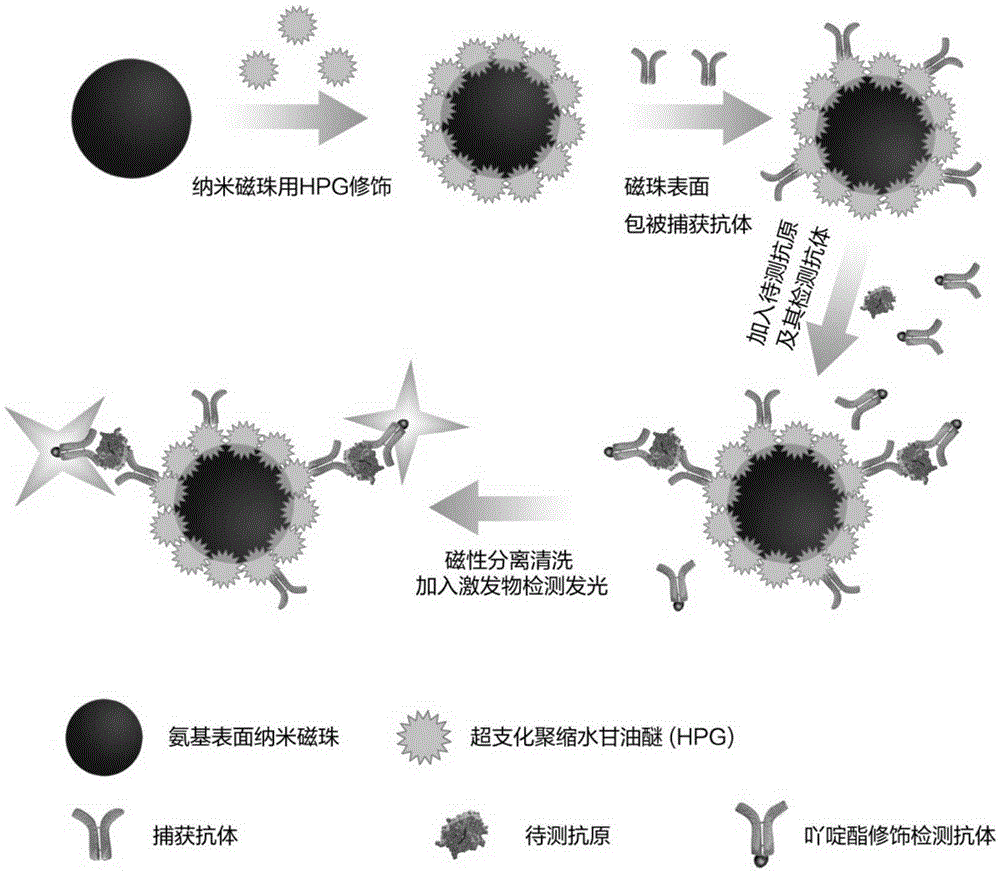
[0052] Example 1 Preparation method of diagnostic reagent based on hyperbranched polyglycidyl ether modified magnetic microspheres
[0053] Preparation of Hyperbranched Polyglycidyl Ether
[0054] In a three-neck flask equipped with mechanical stirring and a micro constant flow pump, add 0.24g trimethylolpropane (TMP) as the initiator of the reaction, using 3.7MCH 3 OK (dissolved in methanol) partially deprotonated the hydroxyl group of TMP, the deprotonated part accounted for 10% of the total part, and the excess methanol was removed by heating and evaporating. Then 50mL of glycidol was slowly added dropwise into the system to initiate polymerization, pay attention to the drop rate of glycidol, as dropping too fast may cause implosion. The reaction temperature was 95°C, and the reaction was carried out for 12 hours.
[0055] After the reaction, the product was dissolved in methanol and passed through a strong acid cation exchange resin (AMBERJET TM 1500HResin) neutralization...
Embodiment 2
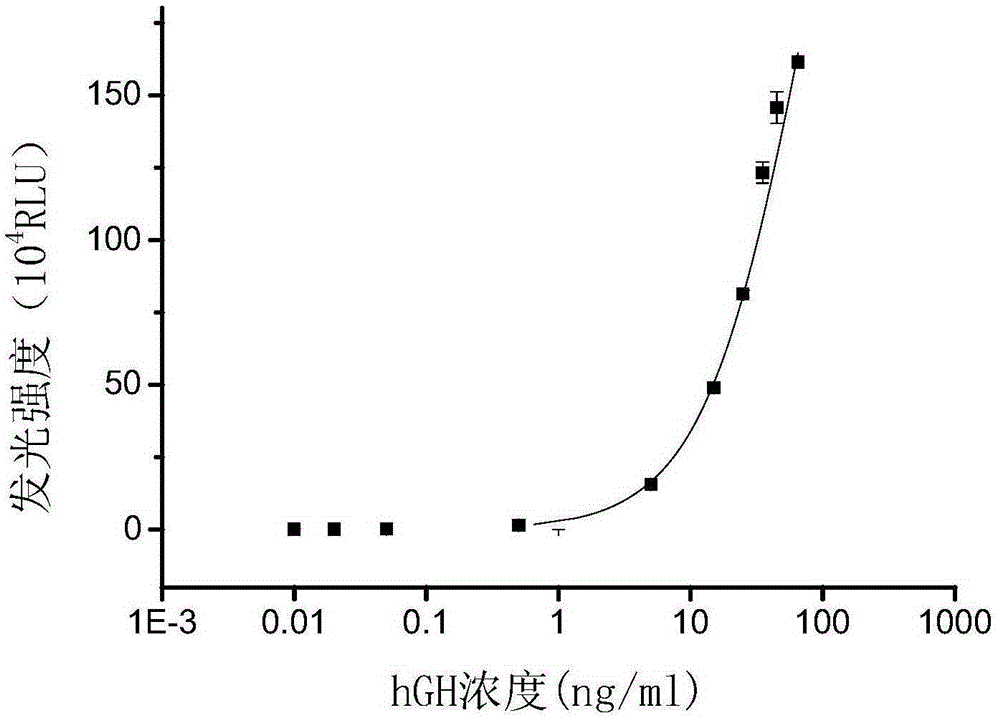
[0062] In Example 2, the commercial carboxyl magnetic microspheres (manufacturer JSR diagnostic reagent, article number MS160 / Carboxyl) used as a control reagent were coupled to the hGH antibody in the same way as above, only commercial carboxyl magnetic microspheres were used instead of hyperbranched polyglycidyl ether Modified magnetic microspheres.
[0063] Labeling of acridinium esters
[0064] Take hGH‐5801 antibody (manufacturer MedixmAB product number 100041) and add it to 50mMPBS buffer (pH=8.0), add 0.5mmol / L acridinium ester, mix, and react at room temperature for 20min in the dark. Use 10mMPBS buffer (pH=6.5) to remove unbound acridinium esters by dialysis, and finally add 50% redistilled glycerol and store at -20°C.
[0065] Chemiluminescence excitation solution system
[0066] Prepare excitation solution 1 and excitation solution 2: excitation solution 1 is 0.2M nitric acid solution containing 0.5% hydrogen peroxide solution; excitation solution 2 is 0.5M sodium...
Embodiment 3
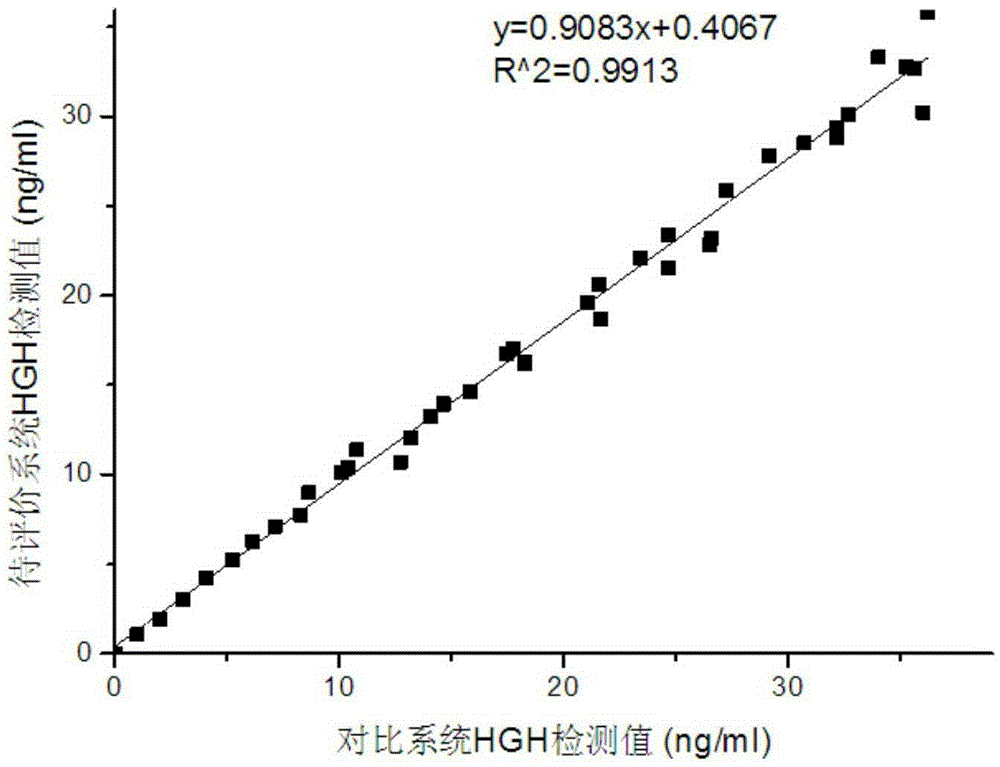
[0099] Validation of diagnostic reagents developed by hyperbranched polyglycidyl ether modified magnetic microspheres in TPO-Ab project using indirect method in chemiluminescence immunoassay
[0100] The hyperbranched polyglycidyl ether-modified nano-magnetic microsphere-TPO antigen (manufacturer Hytest product number 8RTPO) complex was prepared according to the method of Example 1, and was used for the following TPO-Ab chemiluminescence immunoassay. In this example, the coupling method of commercialized carboxyl magnetic microspheres (manufacturer JSR diagnostic reagent, article number MS160 / Carboxyl) and TPO antigen as a control reagent is the same as that in Example 1, only commercial carboxyl magnetic microspheres are used instead of hyperbranched polymeric microspheres. Glycidyl ether modified magnetic microspheres.
[0101] Add hyperbranched polyglycidyl ether-modified nano-magnetic microspheres-TPO antigen complex to 20 μL of the sample to be analyzed, mix well, and inc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com