Preparation method and application of thin-layer nano flaky total-silicon molecular sieve for preparing caprolactam
An all-silicon molecular sieve and nano-sheet technology, applied in the direction of silicon oxide, silicon dioxide, etc., can solve the problems of complex process, low conversion rate, and easy volatility, so as to reduce coking deactivation, improve catalytic activity, and reduce environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
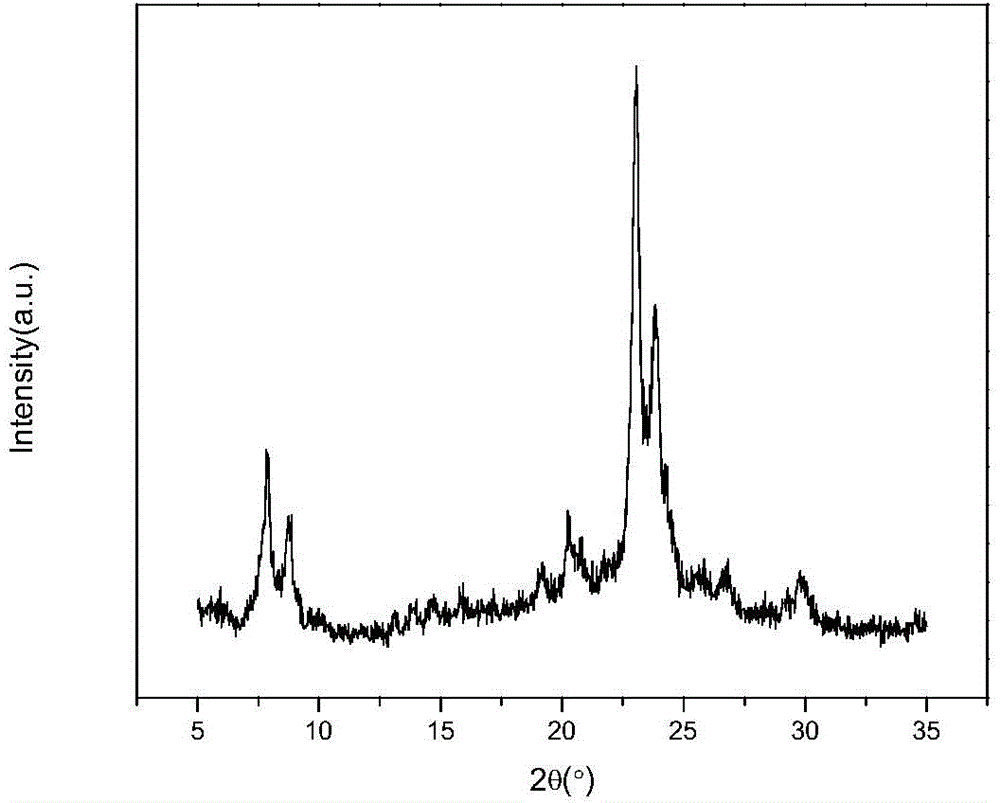
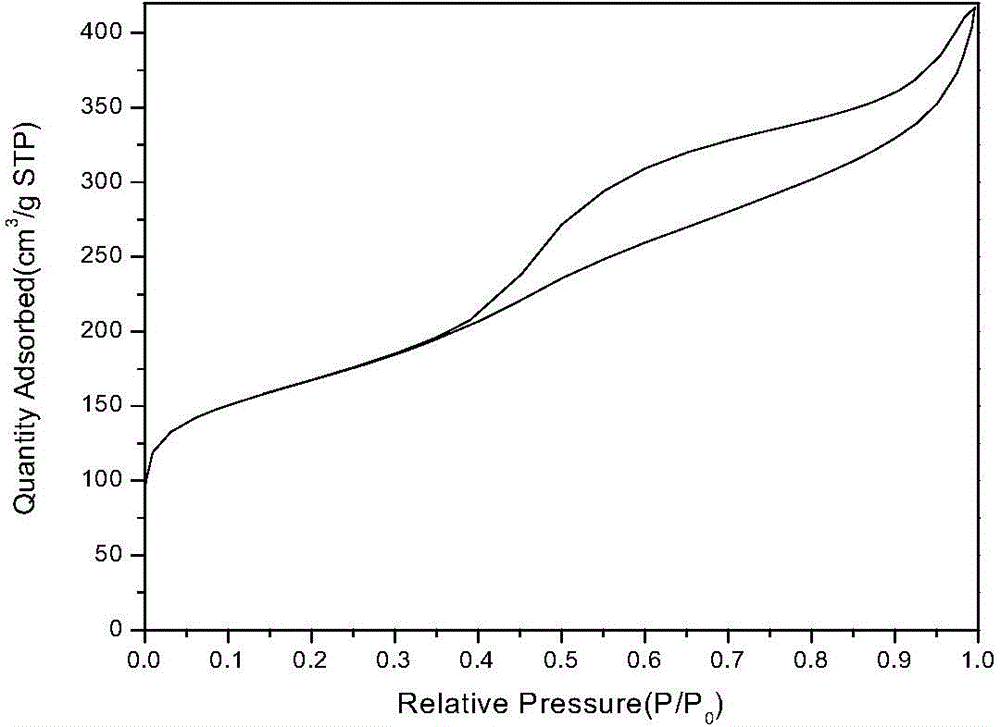
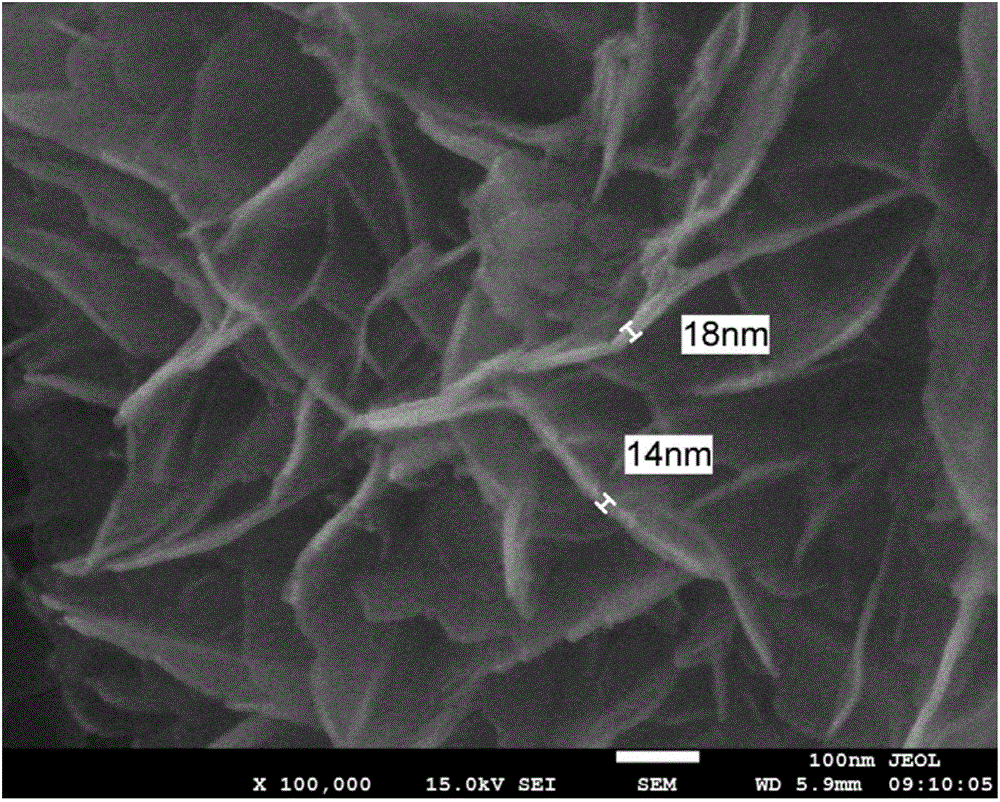
Image
Examples
Embodiment 1
[0042] a. Add 51.6 g of tetramethylhexamethylenediamine, 337 ml of dimethyl carbonate, and 33.3 g of bromooctadecane into a flat-bottomed flask, heat under reflux in a water bath at 50°C for 8 hours while stirring, cool to room temperature, and filter , washed with dimethyl carbonate for 1-2 times, and air-dried to make an intermediate; add 50.5 g of the intermediate, 300 ml of ethanol, and 49.5 g of bromohexane into a flat-bottomed flask, and heat under reflux in an oil bath at 79 °C under stirring 10 hours. After completion, heat up to 85°C, stir and dry to obtain a light yellow translucent solid, cool to room temperature, wash with ether 1-2 times, and obtain template C 18-6-6 Br 2 , the yield is 99%.
[0043] b. Add 4.98 g of ethyl orthosilicate to 3.83 g of tetramethylammonium hydroxide, stir at room temperature for 10 minutes, then add 17 g of deionized water, 2.5 g of ethanol, and 0.85 g of the template prepared in step a above C 18-6-6 Br 2 , after stirring evenly...
Embodiment 2
[0047] a. Add 86 g of tetramethylhexamethylenediamine, 500 ml of diethyl carbonate, and 39 g of docosane bromide into a flat-bottomed flask, heat under reflux in a water bath at 55 °C for 5 hours under stirring, and cool to room temperature. Centrifuge, wash with diethyl carbonate for 1-2 times, air-dry to make the intermediate; add 56.1 g of the intermediate, 300 ml of ethanol, and 33 g of bromohexane into a flat-bottomed flask, and reflux in an oil bath at 79 °C while stirring Heat for 10 hours. After completion, heat up to 85°C, stir and dry to obtain a yellow translucent solid, cool to room temperature, wash with ether 1-2 times, and obtain template C 22-6-6 Br 2 , the yield is 99.2%.
[0048] b. Add 4.98 g of ethyl orthosilicate to 1.28 g of tetramethylammonium hydroxide, stir at room temperature for 15 minutes, then add 14.40 g of deionized water, 2.5 g of ethanol, and 0.78 g of the template prepared in step a above C 18-6-6 Br 2 , after stirring evenly, heat in a w...
Embodiment 3
[0051] a. Add 51.6 g of tetramethylhexamethylenediamine, 350 ml of dimethyl carbonate, and 24.9 g of dodecane bromide into a flat-bottomed flask, heat under reflux in a water bath at 45 °C for 10 hours while stirring, cool to room temperature, and centrifuge , washed 1-2 times with dimethyl carbonate, and air-dried to make an intermediate; add 42.1 g of intermediate, 300 ml of propanol, and 20.55 g of bromobutane into a flat-bottomed flask respectively, and reflux in an oil bath at 85 °C while stirring Heat for 8 hours. After completion, heat up to 90°C, stir and dry, cool to room temperature, and wash with ether 1-2 times to obtain template C 12-6-4 Br 2 , the yield is 99.3%.
[0052] b. Add 4.98 g of ethyl orthosilicate to 1.28 g of tetraethylammonium hydroxide, stir at room temperature for 8 minutes, then add 18.90 g of deionized water, 2.5 g of ethanol, and 0.69 g of the template prepared in step a above C 12-6-4 Br2 , after stirring evenly, heated in a water bath at 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com