Synthesis method of alogliptin benzoate
A synthesis method and technology of benzoic acid, applied in carboxylate salt preparation, organic chemistry, etc., can solve the problems that impurity content is difficult to meet the qualified requirements of raw materials, resulting in reduced yield, high anhydrous requirements, etc., to achieve industrial production and easy Control, less environmental pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, a kind of synthetic method of alogliptin benzoate comprises the following steps:
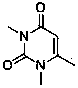
[0043] (1) 2-Cyanobenzyl bromide and 3-methyl-6-chlorouracil were stirred and reacted in toluene in the presence of tri-n-butylamine, cooled and added with water to stir and crystallize, filtered and washed to obtain 2-(6-chloro- 3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-benzonitrile;
[0044] (2) 2-(6-Chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-benzonitrile and (R)-3- Aminopiperidine dihydrochloride and alkali were added to ethanol, stirred and reacted, and after purification, it was salified with benzoic acid to obtain 2-({6-[(3R)-3-aminopiperidin-1-yl]-3- Methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)-benzonitrile monobenzoate.
Embodiment 2
[0045] Example 2, in the step (2) of the synthetic method of alogliptin benzoate described in Example 1, the amount of the substance used (R)-3-aminopiperidine dihydrochloride is 2-( 1.0 to 1.5 times the amount of 6-chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-benzonitrile.
Embodiment 3
[0046] Example 3, in the step (2) of the synthesis method of alogliptin benzoate described in Example 1 or 2, the base is sodium bicarbonate or sodium carbonate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com