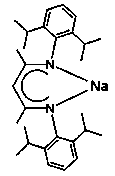
Beta-diketiminate divalent rare earth boron hydrogen complex and preparation method and application thereof
A -diimine-based divalent rare earth, -diimine technology, applied in the field of rare earth metal complexes, can solve the problem of low reduction potential of divalent rare earth metal ions, limiting the catalytic application of divalent rare earth complexes, and divalent rare earth complex complex preparation and other problems, to achieve the effect of simple and controllable reaction process, easy purification, and stable storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example one: [2,6- i pr 2 -(C 6 H 3 )-NC(Me)CHC(Me)N-(C 6 H 3 )-2,6- i pr 2 ] Yb-BH 4 . Synthesis of 2THF
[0040] In the dehydrated and deoxygenated reaction flask, use a syringe to take β-diimine ligand sodium salt ([2,6- i pr 2 -(C 6 H 3 )-NC(Me)CHC(Me)N-(C 6 H 3 )-2,6- i pr 2 ] Na) and equimolar amount of YbCl 3 The THF slurry was reacted with THF slurry at room temperature and stirred for 24 hours to obtain β-diimine ytterbium dichloride quantitatively. The β-diimine ytterbium dichloride (4.30 mmol) and an equimolar amount of sodium borohydride (4.30 mmol) ) After reacting at room temperature for 24 hours, add 1.2 equivalents of sodium potassium alloy with a molar ratio of 9:1, and react at room temperature for 24 hours. The precipitate was removed by centrifugation, the clear solution was transferred to another crystallization flask, concentrated appropriately, the flask was sealed, and crystallized at room temperature to obtain black crystals of 2.36 (3.14 mmol) with...
Embodiment 2
[0041] Example two: [2,6- i pr 2 -(C 6 H 3 )-NC(Me)CHC(Me)N-(C 6 H 3 )-2,6- i pr 2 ] Sm-BH 4 . Synthesis of 2THF
[0042] In the dehydrated and deoxygenated reaction flask, use a syringe to take β-diimine ligand sodium salt ([2,6- i pr 2 -(C 6 H 3 )-NC(Me)CHC(Me)N-(C 6 H 3 )-2,6- i pr 2 ] Na) and equimolar amount of SmCl 3 The THF slurry was reacted with THF slurry at room temperature and stirred for 24 hours to obtain β-diimine samarium dichloride quantitatively. The β-diimine samarium dichloride (4.84 mmol) and an equimolar amount of sodium borohydride (4.84 mmol) ) After 24 hours of reaction at room temperature, 1.2 equivalents of sodium potassium alloy with a molar ratio of 9:1 are added, and the reaction is performed at room temperature for 24 hours. The precipitate was removed by centrifugation, the clear solution was transferred to another crystallization flask, concentrated appropriately, the flask was sealed, and crystallized at room temperature to obtain 2.76 g (3.78 mmo...
Embodiment 3
[0043] Embodiment Three: [2,6- i pr 2 -(C 6 H 3 )-NC(Me)CHC(Me)N-(C 6 H 3 )-2,6- i pr 2 ] Sm-BH 4 . 2THF-catalyzed synthesis of α-hydroxy phosphate from benzaldehyde and diethyl phosphite
[0044] In the reaction flask that has been dehydrated and deoxygenated, the catalyst is added under the protection of argon [2,6- i pr 2 -(C 6 H 3 )-NC(Me)CHC Me)N-(C 6 H 3 )-2,6- i pr 2 ] Sm-BH 4 . 2THF (0.01 mmol, 0.0073 g), then use a syringe to add diethyl phosphite (1.55 mL, 12 mmol), then stir at room temperature for 10 minutes, then use a syringe to add benzaldehyde (1.01 mL, 10 mmol), and react for 5 minutes After that, the reaction was terminated with ethyl acetate and dissolved with an appropriate amount of ethyl acetate. The solvent was removed by rotary evaporation. The remaining solid was washed with n-hexane (4×5 mL) to obtain the corresponding α-hydroxy phosphate, C 6 H 4 CHOHPO(OCH 2 CH 3 ) 2 , 2.2213 g, 91% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com