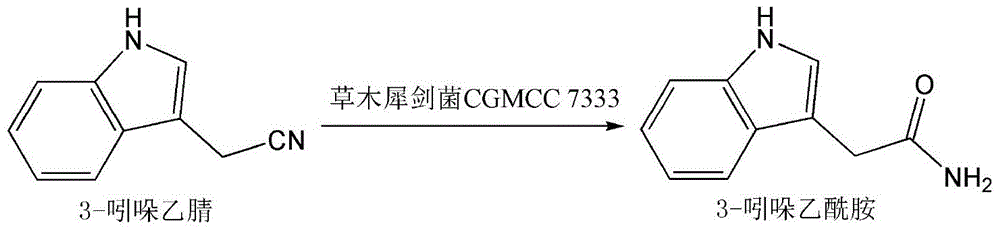
Application of M. frugosa in the biotransformation of 3-indoleacetonitrile to synthesize 3-indoleacetamide
A technology of sword rhinoceros oleracea and indoleacetamide is applied in the field of microorganisms to achieve the effects of simple process, easy cultivation and high transformation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Streak Mushroom CGMCC7333 on LB solid agar medium. The composition of LB medium is: peptone 10g / L, yeast extract 5g / L, NaCl 10g / L, pH adjusted to 7.0, and 20g / L agar powder is added to prepare solid medium. Cultivation was based on autoclaving at 115°C for 20 minutes. After a single colony grows on the solid plate, pick a single colony and inoculate it into 10mL LB liquid medium with a final concentration of 0.1mmol / L cobalt chloride, culture at 30°C, shake at 220rpm for 24h, then centrifuge at 8000rpm for 10min to collect The cells were suspended in 0.05 mol / L potassium phosphate buffer at pH 7.5, and the cell concentration (OD600) was adjusted to 5.0. Take 5 mL of the above cell suspension in a 50 mL centrifuge tube, and add 0.5 mg of 3-indoleacetonitrile (concentration: 0.5 g / L). The centrifuge tube was reacted in a shaker at 30°C and 220rpm. After 12 hours of reaction, samples were taken, centrifuged at 8000rpm for 10 minutes, and the supernatant was ta...
Embodiment 2
[0013] Example 2: Use a sterile toothpick to pick up a ring of activated Sword Mushroom CGMCC7333 and place it in a 100mL Erlenmeyer flask containing 20mL of liquid LB medium. After culturing for 16 hours, take 1 mL and inoculate it in 100 mL of LB liquid medium containing 0.1 mmol / L cobalt chloride at 30°C, shake at 220 rpm for 12 hours, centrifuge at 10,000 rpm for 10 min, collect the cells and suspend them in 0.05 mol / L of pH 7.5 In potassium phosphate buffer, the cell concentration (OD600) was adjusted to 5.0. The content of 3-indoleacetamide was sampled and determined after 12 hours of conversion, and the result showed that under the above conditions, the concentration of 3-indoleacetamide generated was 0.76 g / L.
Embodiment 3
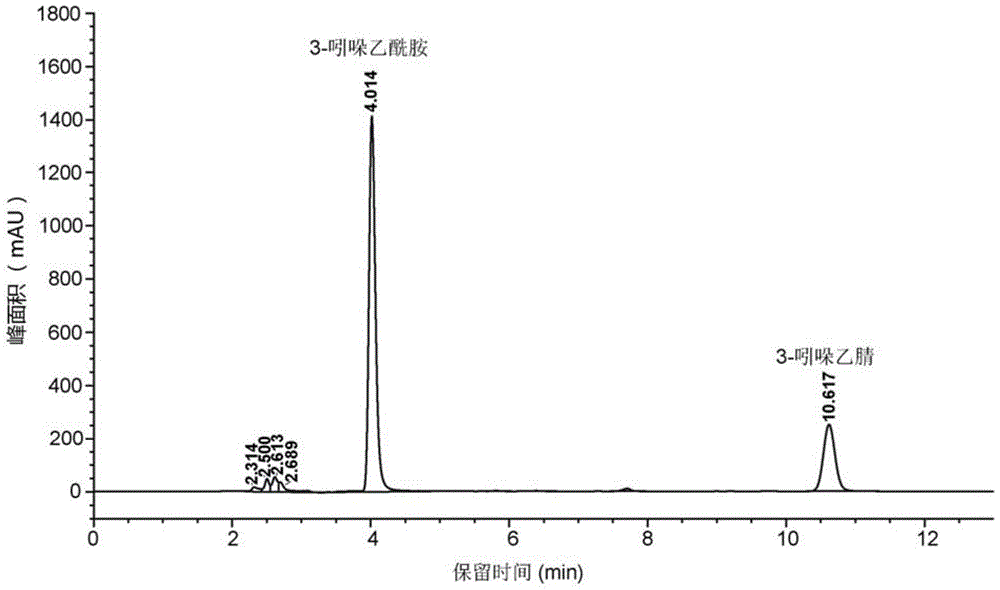
[0014] Example three: basically the same as Example two, the conversion time is 24h, and the HPLC quantitative analysis results show that the concentration of the generated 3-indoleacetamide is 1.03g / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com