Method for producing cobra CT and PLA2 in baculovirus-insect expression system
A technology of insect expression and baculovirus, applied in the field of genetic engineering, can solve problems affecting the function of CT and PLA2 proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0204] Embodiment 1: Guangxi Cobra CT and PLA 2 Obtaining the mature peptide sequence
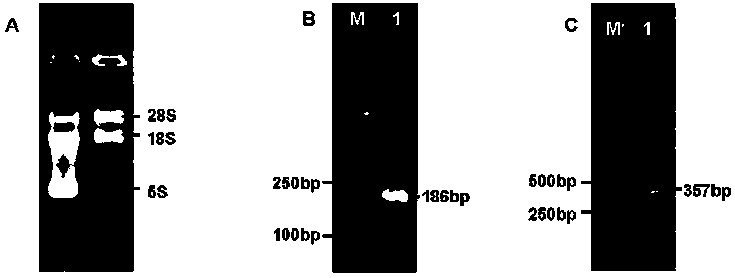
[0205] 1. Extraction of total RNAR from snake venom glands
[0206] The specific steps of Guangxi cobra venom total RNA extraction are as follows:
[0207] (1) Homogenization treatment Put 50-100 mg of Guangxi cobra venom glands into a sterilized and pre-cooled mortar with liquid nitrogen, add an appropriate amount of liquid nitrogen, grind the tissue fully with a grinding pestle, add the lysate RZ after grinding, The sample volume should not exceed one-tenth of the RZ volume of the lysate.
[0208] (2) Place the homogenized sample at 30°C for 5 min to separate the nucleic acid and protein complexes.
[0209] (3) Add 200 μL of pre-cooled chloroform, cover the tube cap, oscillate for 15 s, and place at room temperature for 3 min.
[0210] (4) After ultracentrifugation at 12000 rpm at 4 ℃ for 10 min, the sample will be divided into three layers: the middle layer, the colorless aqueous ph...
Embodiment 2
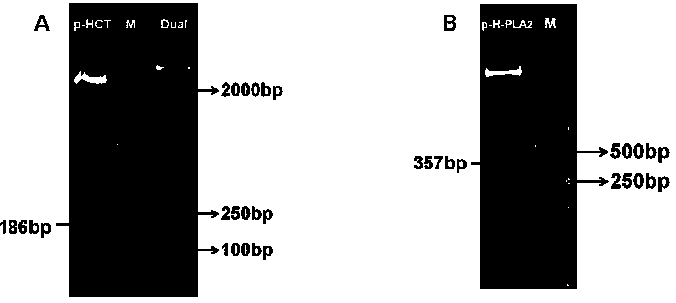
[0228] Example 2: CT and PLA 2 Construction of bacmid
[0229] 1. CT and PLA2 Recovery of PCR products
[0230] Specific steps are as follows:
[0231] (1) Equilibrium column: Add 500 μL of equilibrium solution BL to the adsorption column CA2, ultracentrifuge at 12,000 rpm for 1 min, discard the waste liquid, and put the adsorption column CA2 back into the collection tube.
[0232] (2) Cut out a single target nucleic acid band, cut the cut gel into pieces, put it into a clean EP tube, place it on a balance and weigh it.
[0233] (3) Add 3 times the volume of sol solution PN to the above-mentioned EP tube. Note: The calculation method for the volume of glue is 0.1 g=0.1 mL. Then put it in a constant temperature water bath at 50 °C for 10 min, and gently turn the EP tube up and down every 2 min until the glue block is completely dissolved.
[0234] (4) Add the sol solution obtained in step 3 into the adsorption column CA2, place at 25 °C for 2 min, then ultracentrifuge at 1...
Embodiment 3
[0252] Example 3: CT and PLA 2 Construction of Insect Virus Recombinant Plasmid
[0253] 1. Competent Cell Preparation of Escherichia coli DH10Bac
[0254] (1) Spread Escherichia coli DH10Bac cells on LB (Kan+) solid plates, screen freshly activated DH10Bac single colonies, and inoculate the colonies in a 250 mL Erlenmeyer flask containing 2 mL LB liquid medium (Kan+), 37 Cultivate with shaking overnight at ℃, inoculate 0.5 mL of the overnight culture solution into a new 50 mL of the same medium the next day, and continue shaking to OD 600 The value reaches around 0.6.
[0255] (2) Under aseptic conditions, transfer the above bacterial solution to a 50 mL EP tube pre-cooled at 0°C, place on ice for 10 minutes, cool the bacterial solution to 0°C, centrifuge at 4,000 rpm for 10 minutes at 4°C, and discard the supernatant , invert the tube for several minutes to allow residual medium to flow out.
[0256] (3) Use 10 mL of 0.1 M CaCl pre-cooled at 0 ℃ 2 Resuspend the cell pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com