Nadroparin calcium preparation technology
A technology of nadroparin calcium and preparation process, applied in the field of nadroparin calcium production technology, can solve the problems of incomplete calcium transfer, cumbersome operation of calcium transfer process, long time, etc., and achieve complete calcium transfer, wide range of use, cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
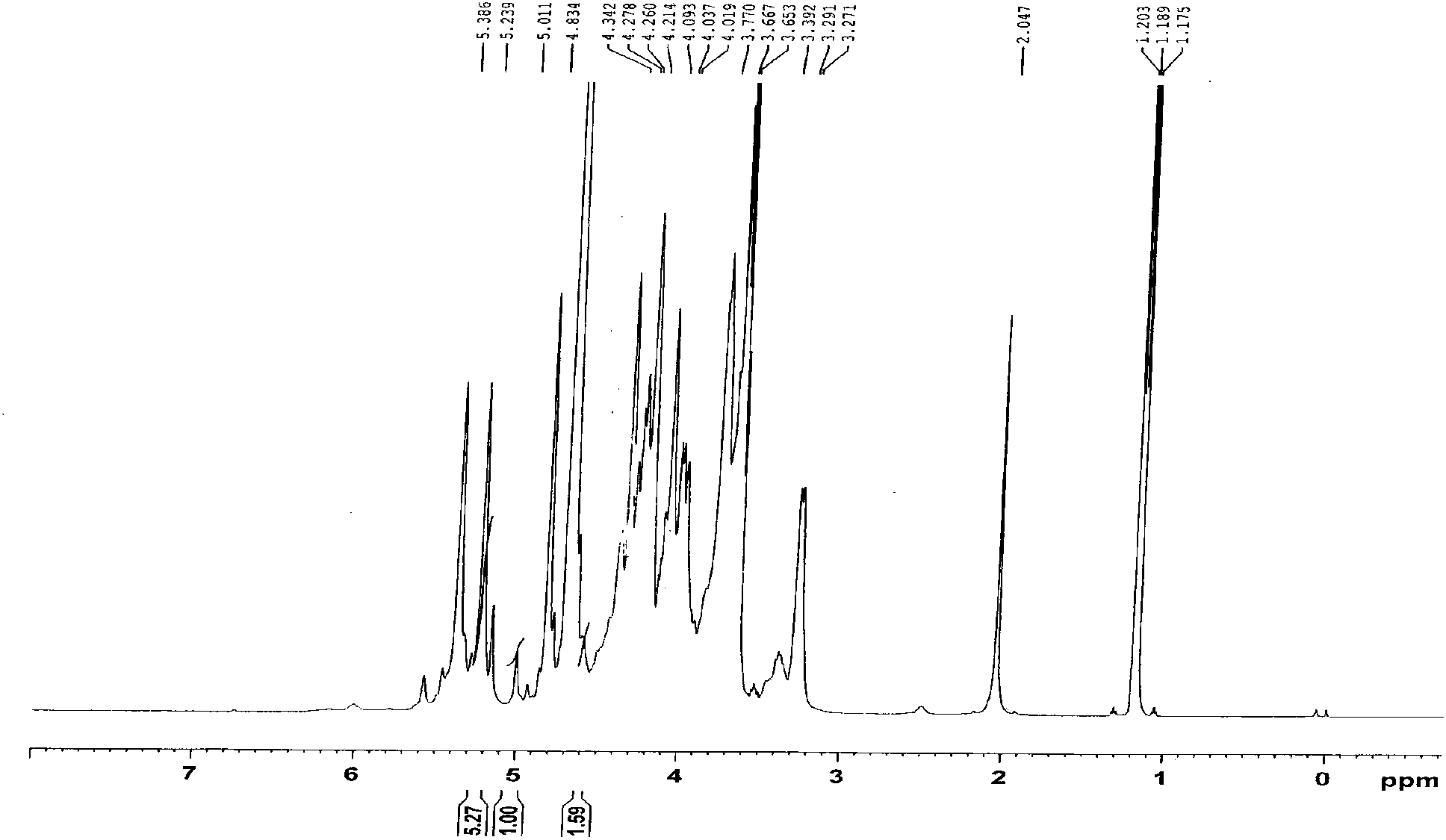
Image
Examples
Embodiment 1
[0033] a. Preparation of degradation solution
[0034] Add 3Kg of heparin sodium into 15L~24L of pure water and stir to dissolve, then adjust the pH of the solution to 2~3 with hydrochloric acid, then add 60g of sodium nitrite, stir and react at 10~30°C for 2~5h, then cool at 2~10°C Stand still for 20-24 hours;
[0035] b. Preparation of reducing solution
[0036] In the degradation solution prepared in step ②, use sodium hydroxide solution to adjust the pH of the solution to 9-11, add 18g of sodium borohydride for reduction for 10-16 hours, and use sodium hydroxide to adjust the pH of the solution to 6.5-7.0 after the reaction get the reducing solution;
[0037] c. Collect the sediment
[0038] Add 95% volume concentration of ethanol to the reducing solution for precipitation and collect the precipitate;
[0039] d. Oxidation
[0040] After adding pure water to the collected precipitate and stirring to dissolve, add 0.2% to 1.0% mass concentration of hydrogen peroxide to...
Embodiment 2
[0049] Example 2: The difference between this example and Example 1 is that the sodium heparin is 2Kg, the sodium nitrite is 40g, and the sodium borohydride is 20g.
Embodiment 3
[0050] Example 3: The difference between this example and Example 1 is that the sodium heparin is 4Kg, the sodium nitrite is 80g, and the sodium borohydride is 40g.
[0051] Instance detection results
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com