Cereus sinensis verrill soluble cell peptide CCT-I and separating and purifying method and application thereof
A technology of CCT-I and separation method, which is applied in the field of CCT-I, a sea anemone cytolytic polypeptide and its separation and purification, can solve the problem of the difficulty of separation and purification of sea anemone toxin, the separation and purification of cytolytic toxin, and the research on its composition and properties. There have been no reports, activity reduction or even loss and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 Separation of the Cytolytic Polypeptide of Sea Anemone chinensis
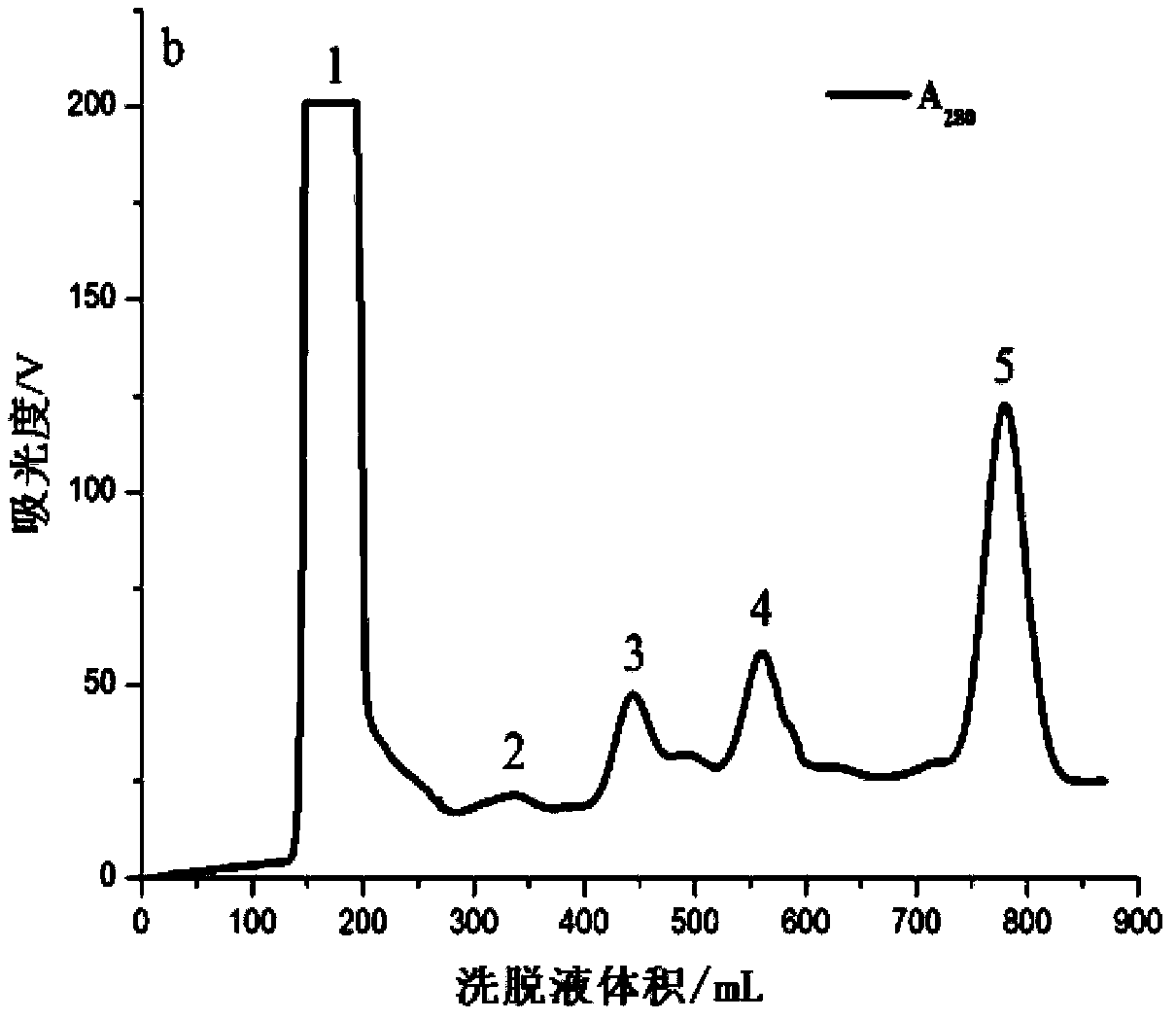
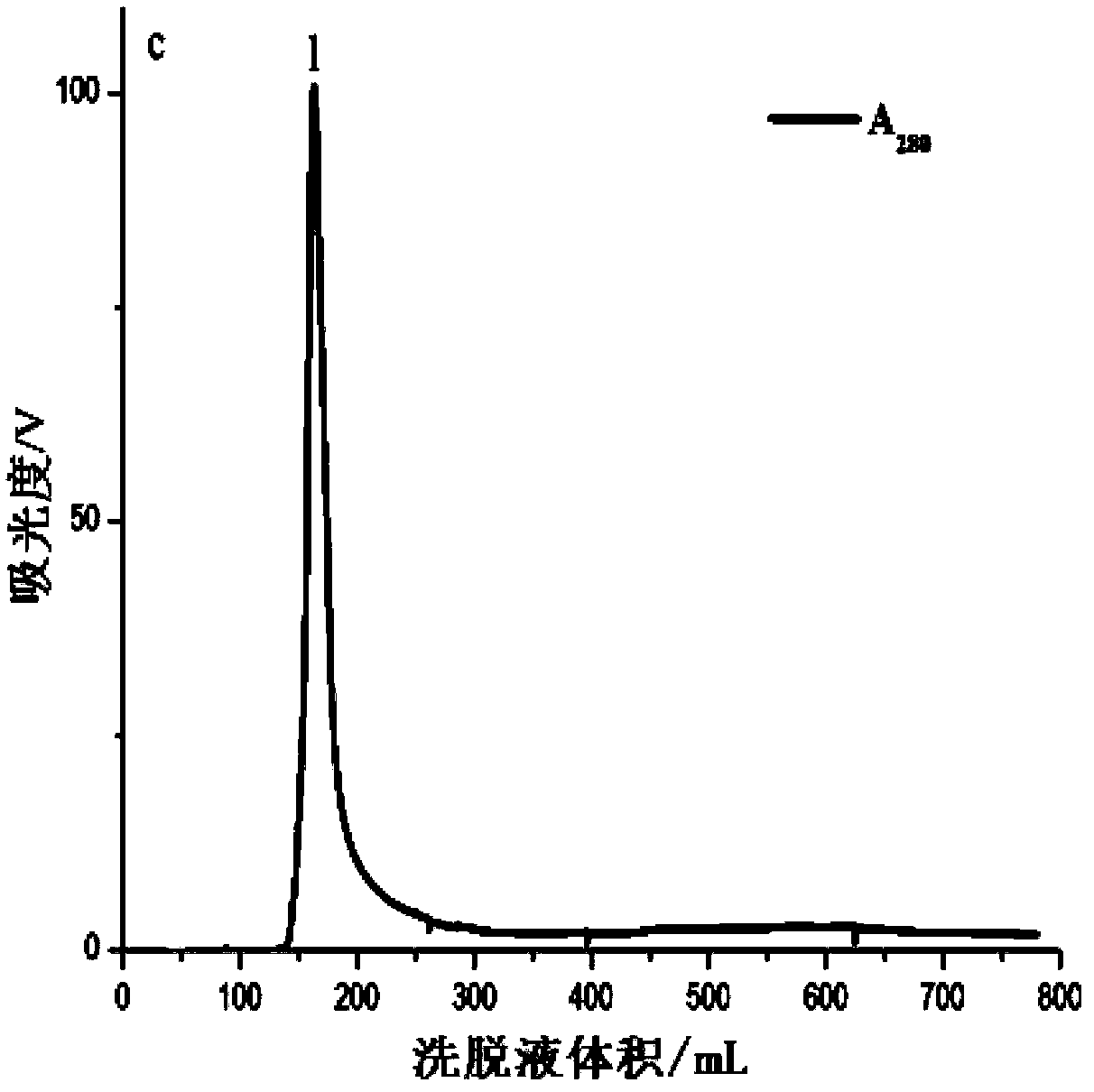
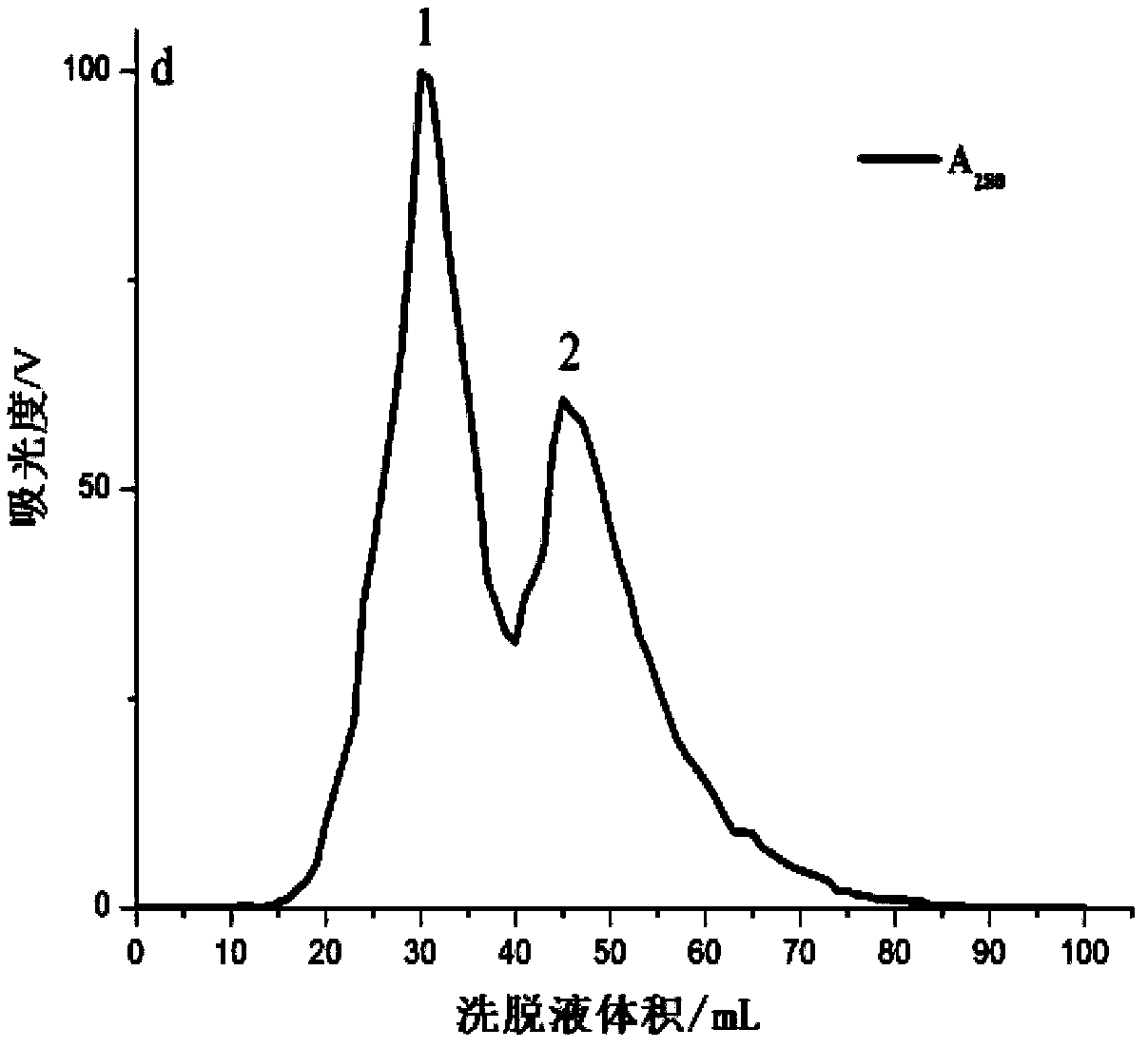
[0035] In the first step, the sea anemone is crudely prepared. Cut off the tentacles of the live Anemone chinensis, and quickly freeze them in a -80°C refrigerator. Before preparation, take out the tentacles that have been frozen and thawed twice, and after thawing at room temperature, weigh 20g accurately and cut them into pieces with scissors. After fully homogenizing with a glass homogenizer, add a certain amount of ultrapure water according to the material-to-liquid ratio of 1:5, seal it with a parafilm, and place it in a refrigerator at 4°C for 2 hours. During this period, stir once every 15 minutes, pay attention to stirring gently so as not to generate a lot of foam. Then centrifuge at 4°C and 10000r / min for 1 hour, take out the supernatant, and then centrifuge for 1 hour under the same condition, and the obtained supernatant is the sea anemone crude venom. The protein concentration...
Embodiment 2
[0039] The detection of embodiment 2 hemolytic activity detection
[0040] The first step is blood sample preparation. Healthy adult SD rats were taken, blood was collected from the heart, the blood was stored in an anticoagulant tube, and stored at 4°C for future use.
[0041] In the second step, 2% erythrocyte suspension of SD rats was prepared. A certain amount of SD rat blood was taken out from the anticoagulant tube, and 10 mmol·L -1 Phosphate buffered saline (PBS pH=7.0 containing 0.15mol·L -1 Sodium chloride) washing, 1500r·min at 4°C -1 Centrifuge for 5 minutes, discard the supernatant, and repeat 4 to 5 times until the supernatant is clear and transparent without red. The red blood cells are diluted with PBS to a 2% red blood cell suspension, and stored at 4°C for later use.
[0042] The third step is the detection of hemolytic activity. Add 200 μL of CCT-I solutions of different concentrations to 200 μL of 2% erythrocyte suspension, then dilute to 1 mL with PBS,...
Embodiment 3
[0046] Example 3 Identification of Cytolytic Polypeptide CCT-I of Sea Anemone chinensis
[0047] The first step is SDS-PAGE electrophoresis to check the purity of CCT-I and estimate the molecular weight. The sample was mixed with 5× loading buffer (v:v=4:1) and boiled at 95°C for 5 minutes, then separated with 5% stacking gel and 12% separating gel, and finally fixed and stained with Coomassie brilliant blue R-250, Molecular weights are determined by comparison to standard proteins. The standard proteins contained in the standard protein marker are: phosphatase b 97.2kDa, bovine serum albumin 66.4kDa, ovalbumin 44.3kDa, carbonic anhydrase 29.0kDa, trypsin inhibitor 20.1kDa and lysozyme 14.3kDa. The results show that CCT-I mainly contains two protein bands, the purity is high, and the molecular weight is about 80kDa ( Figure 4 ).
[0048] In the second step, the purity of CCT-I is checked by HPLC. CCT-I was dissolved in 10% acetonitrile (acetonitrile: 0.1% TFA = 1:9), filt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com