Dihydro pyrrolidone derivative and application of same in preparing antitumor drugs
A technology for dihydropyrrolidone and antitumor drugs, which is applied in the preparation of application fields as a variety of tumor suppressor drugs, can solve problems such as the inability to meet the needs of cancer patients and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
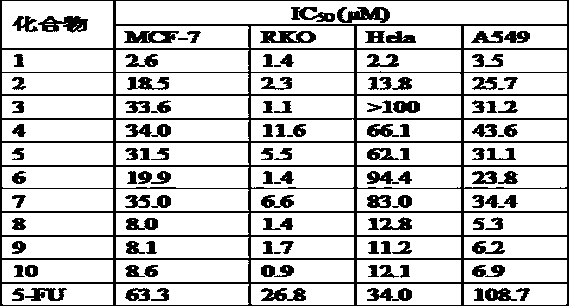
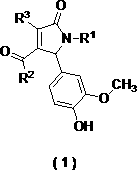
[0057] 2,5-Dihydro-1-(3-trifluoromethylphenyl)2-(3-methoxy-4-hydroxyphenyl)-4-(3-trifluoromethylphenylamino)-5-oxo- 1 H-Methyl 4-(3-(trifluoromethyl)phenylamino)-1-(3-(trifluoromethyl)phenyl)-2,5-dihydro-2-(4-hydroxy-3-methoxyphenyl) -5-oxo-1 H -pyrrole-3-carboxylate Compound 1) 54% yield, yellow solid, mp: 121–122 ºC; IR(KBr) ν max = 3783, 3354, 2923, 2851, 2309, 1701, 1640, 1451, 1330, 1273, 1167, 1124, 1028, 881, 785, 688, 532; 1 H NMR (400 MHz, CDCl 3 ) δ =8.28 (s, 1H), 7.80(s, 1H), 7.71 (d, J = 7.3 Hz, 1H), 7.53–7.35 (m, 6H), 6.83 (s, 2H), 6.66 (s,1H), 5.83 (s, 1H), 5.61 (s, 1H), 3.83 (s, 3H) , 3.63 (s, 3H) ppm; 13 C NMR (101MHz, CDCl 3 ) δ =164.4, 163.7, 146.7, 145.7, 141.1, 138.9, 136.9, 131.2, 130.9,129.4, 129.0, 127.1, 126.0, 125.6, 122.2, 121.3, 121.1, 119.3, 119.2, 119.2,119.2, 114.5, 111.4, 109.2, 63.0 , 55.9, 51.4 ppm; HR-ESI-MS for C 27 h 20 f 6 N 2 o 5 ([M+H] + ) Calcd: 567.1340; Found: 567.1340.
Embodiment 2
[0059] 2,5-Dihydro-1-phenyl-2-(3-methoxy-4-hydroxyphenyl)-4-phenylamino-5-oxo-1 H - Ethyl pyrrole-3-carboxylate
[0060] (ethyl 2,5-dihydro-2-(4-hydroxy-3-methoxyphenyl)-5-oxo-1-phenyl-4-(phenyl-amino)-1 H -
[0061] pyrrole-3-carboxylate compound 2) 58% yield, yellow solid, mp: 107– 108 ºC;IR (KBr) ν max = 3294, 2929, 1693, 1599, 1507, 1375, 1231, 1122, 1032, 758,692, 506; 1 H NMR (400 MHz, CDCl 3 ) δ = 8.19 (s, 1H), 7.48 (d, J = 8.0 Hz, 2H),7.40 –7.05 (m, 8H), 6.84 (m, 2H), 6.65 (s, 1H), 5.79 (s, 1H), 5.56 (s, 1H),4.05 (dt, J = 6.7, 4.5 Hz, 2H), 3.81 (s, 3H), 1.06 (t, J = 7.1 Hz, 3H) ppm; 13 C NMR (101 MHz, CDCl 3 ) δ = 164.4, 164.0, 146.5, 145.4, 141.6, 138.7, 136.5,128.8, 128.4, 125.7, 124.5, 122.5, 121.6, 114.1, 109.1,63.2, 55.9, 53.8, 13.8 PPM; HR- ESI-MS for C 26 h 24 N 2 o 5 ([M+H] + ) Calcd: 445.1758; Found: 445.1755.
Embodiment 3
[0063] 2,5-Dihydro-1-phenyl-2-(3-methoxy-4-hydroxyphenyl)-4-phenylamino-5-oxo-1 H -Methyl 2,5-dihydro-2-(4-hydroxy-3-methoxyphenyl)-5-oxo-1-phenyl-4-(phenyl-amino)-1 H -
[0064] pyrrole-3-carboxylate compound 3) 54% yield, yellow solid, mp: 183– 184 ºC; 1 H NMR (400 MHz, CDCl 3 ) δ = 8.16 (s, 1H), 7.47 (d, J = 7.9 Hz, 2H), 7.40– 7.06(m, 8H), 6.83 (m, 2H), 6.65 (s, 1H), 5.78 (s, 1H), 5.56 (s, 1H), 3.81 (s, 3H) , 3.58 (s, 3H) ppm; 13 C NMR (101 MHz, CDCl 3 ) δ = 164.7, 164.0, 146.6, 145.4, 141.8, 138.6, 136.5, 128.8, 128.4, 128.3, 125.8, 124.7, 122.9, 122.8, 121.3, 114.2, 109.4, 109.2, 5.5 pm
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com