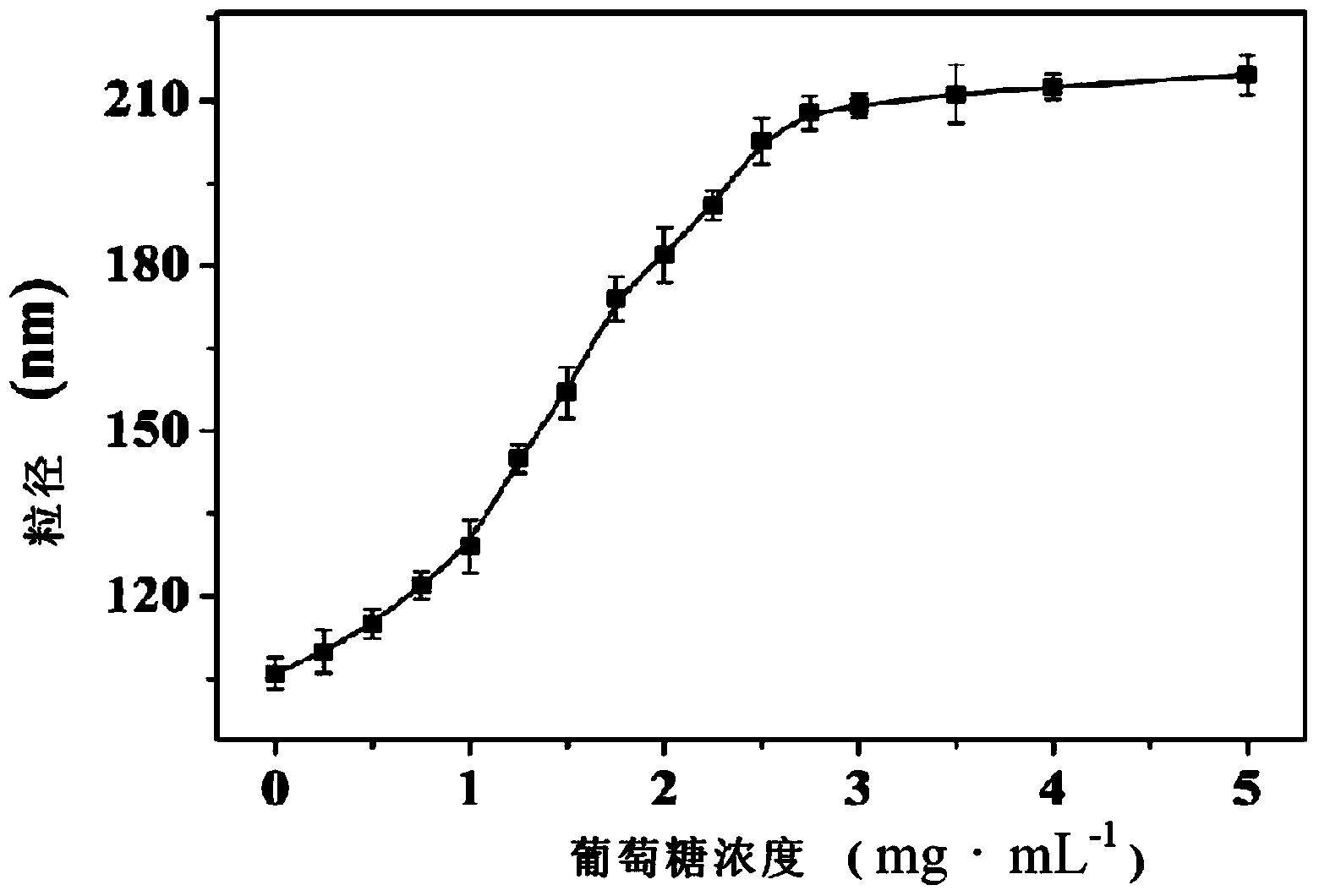
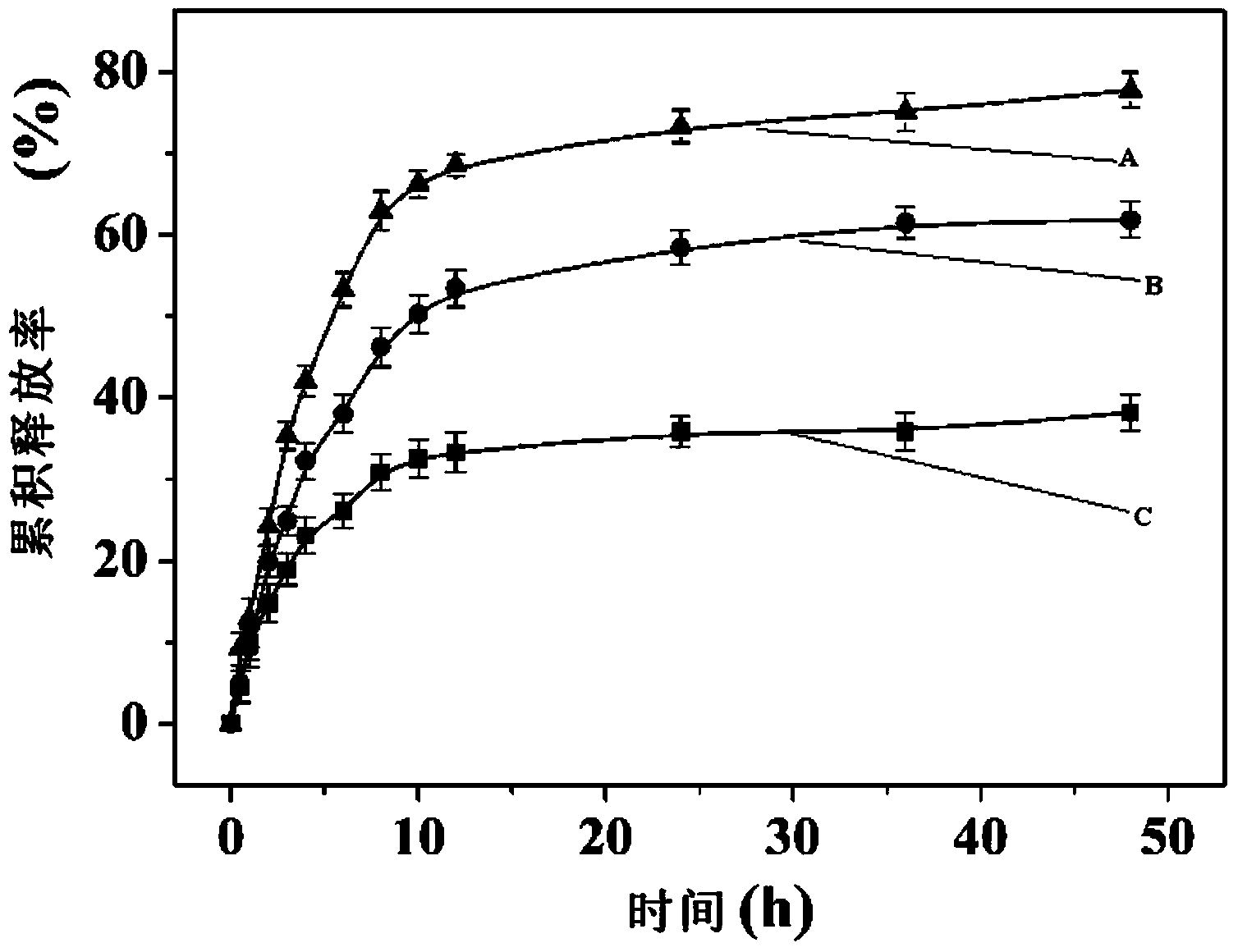
Polymer, glucose-sensitive nanogel, glucose-sensitive drug loading nanogel and preparation methods thereof
A glucose-sensitive, nano-gel technology, applied in the field of polymers, can solve the problems of poor biodegradability and biocompatibility of glucose-sensitive nano-gels, limiting applications, etc., achieve good biodegradability, facilitate rapid release, and improve curative effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
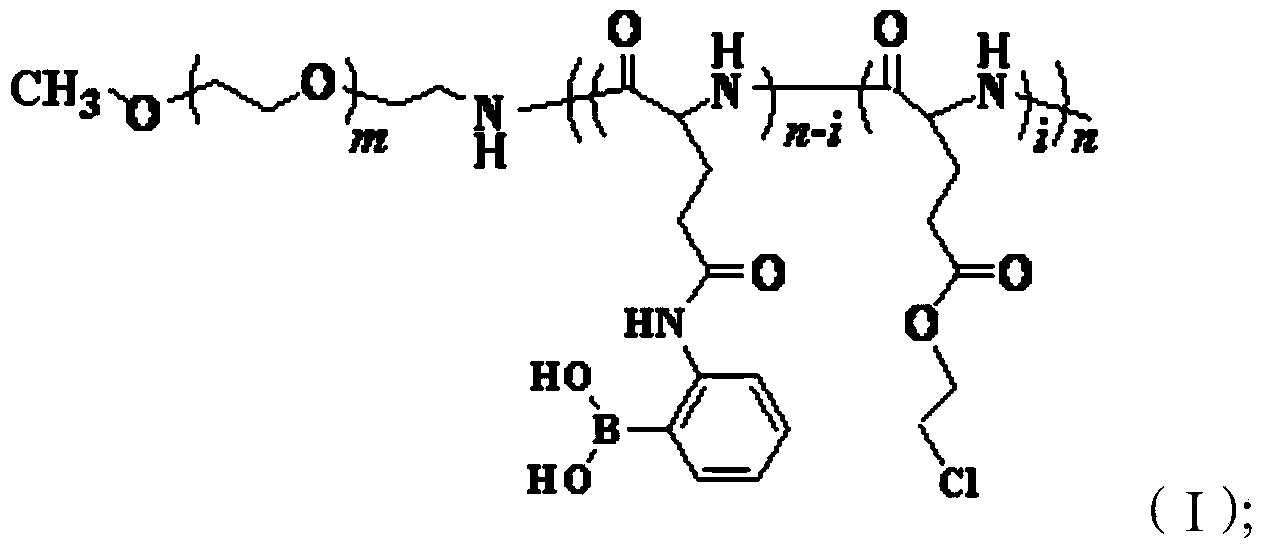
[0037] The invention provides a method for preparing a polymer represented by formula (I), comprising the following steps:
[0038] reacting the polymer represented by formula (II) and 3-aminophenylboronic acid under the action of a condensing agent to obtain the polymer represented by formula (I);
[0039]
[0040]
[0041] Wherein, m is the degree of polymerization, 55≤m≤250, preferably 100≤m≤200, more preferably 110≤m≤150; n is the degree of polymerization, 20≤n≤150, preferably 30≤n≤120, more preferably Preferably 40≤n≤100; 0.05≤i / n<1, preferably 0.1≤i / n<0.7, more preferably 0.2≤i / n≤0.6.
[0042]In the present invention, the polymer represented by formula (II) is reacted with 3-aminophenylboronic acid under the action of a condensing agent to obtain the polymer represented by formula (I). In the reaction, the polymer represented by formula (II) is preferably firstly activated with a condensing agent in an organic solvent, and then reacted with 3-aminophenylboronic ac...
Embodiment 1
[0081] After 25.0 g of polyethylene glycol monomethyl ether with a molecular weight of 5000 was azeotroped to remove water with toluene, it was dissolved in 150.0 mL of anhydrous dichloromethane, and 3.5 mL of triethylamine was added at 0°C under anhydrous conditions, and added dropwise React with 8.0 mL of methanesulfonyl chloride, react at 0°C for 2 hours, return to 25°C, and continue to react for 48 hours under stirring with a stirrer. Dry under vacuum for 24 hours to obtain polyethylene glycol monomethyl ether methanesulfonate.
[0082] Dissolve 3.0 g of the above-prepared polyethylene glycol monomethyl ether methanesulfonate and 1.0 g of ammonium chloride in 80.0 mL of ammonia water with a mass concentration of 25%, and react at 25°C for 72 hours. After the reaction, the amino-terminated polyol Ethylene glycol monomethyl ether, extract the aminated polyethylene glycol monomethyl ether with dichloromethane, wash with 4% sodium chloride aqueous solution, settle ether, filte...
Embodiment 2
[0084] Add 1.041 g (0.208 mmol) of amino-terminated polyethylene glycol monomethyl ether hydrochloride (mPEG-NH 2 HCl), using toluene to azeotropically remove water and then dissolving with anhydrous N,N-dimethylformamide to obtain amino-terminated polyethylene glycol monomethyl ether hydrochloride solution.
[0085] 3.2854g (12.48mmol) of the compound (BLG-NCA) with the structure of the formula (III) and 0.794g (4.16mmol) of the compound (CELG-NCA) with the structure of the formula (IV) were treated with anhydrous N,N-dimethyl Formamide was dissolved and added to the amino-terminated polyethylene glycol monomethyl ether hydrochloride solution, and the reaction was carried out at 25°C under stirring with a stirrer. The reaction time was 72 hours. After the reaction was completed, the solution was poured into the volume Sedimentation in diethyl ether 10 times the amount of the solvent, filtration, washing, and vacuum drying at 25°C for 24 hours to obtain a benzyl alcohol-protec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com