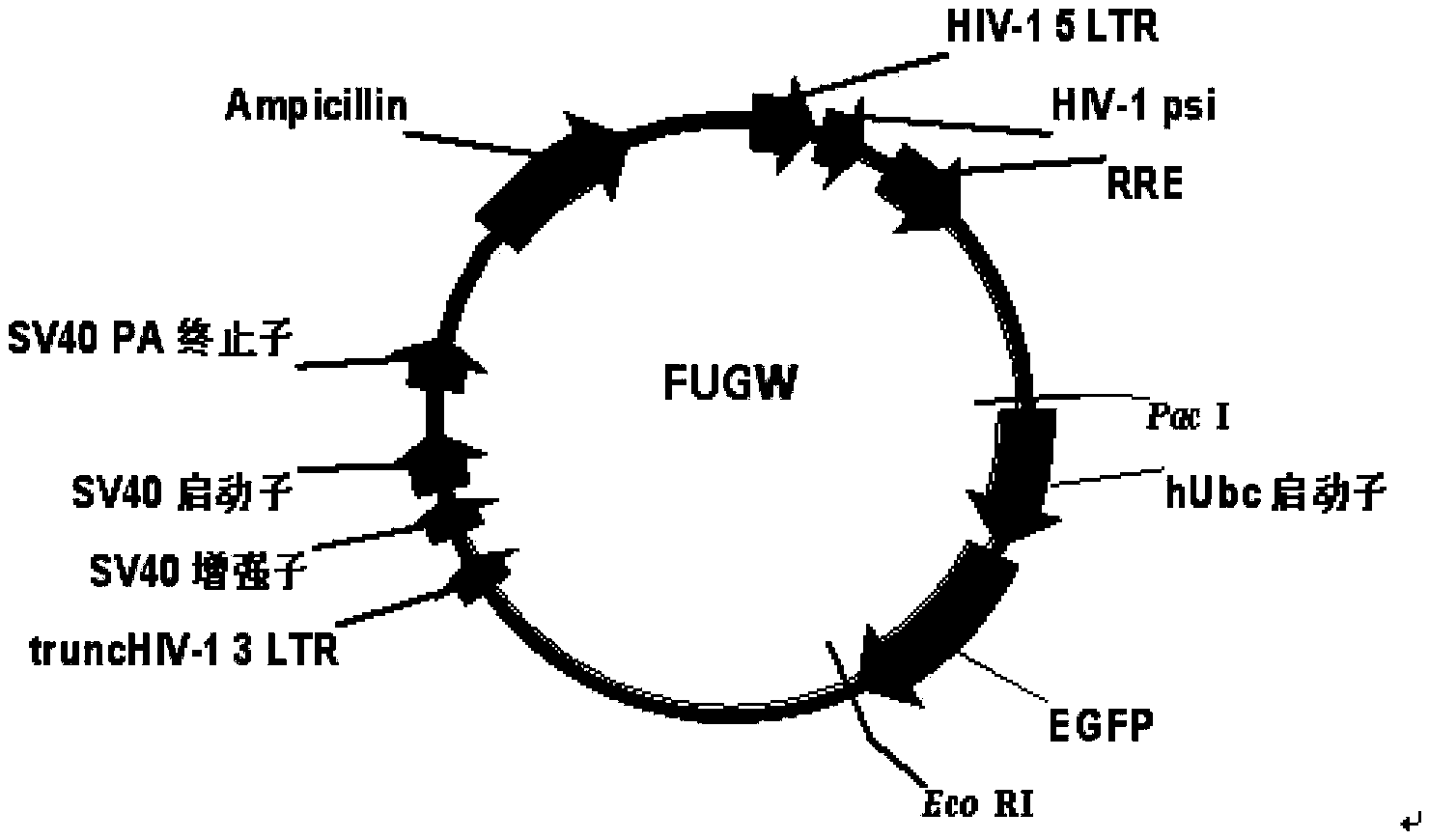
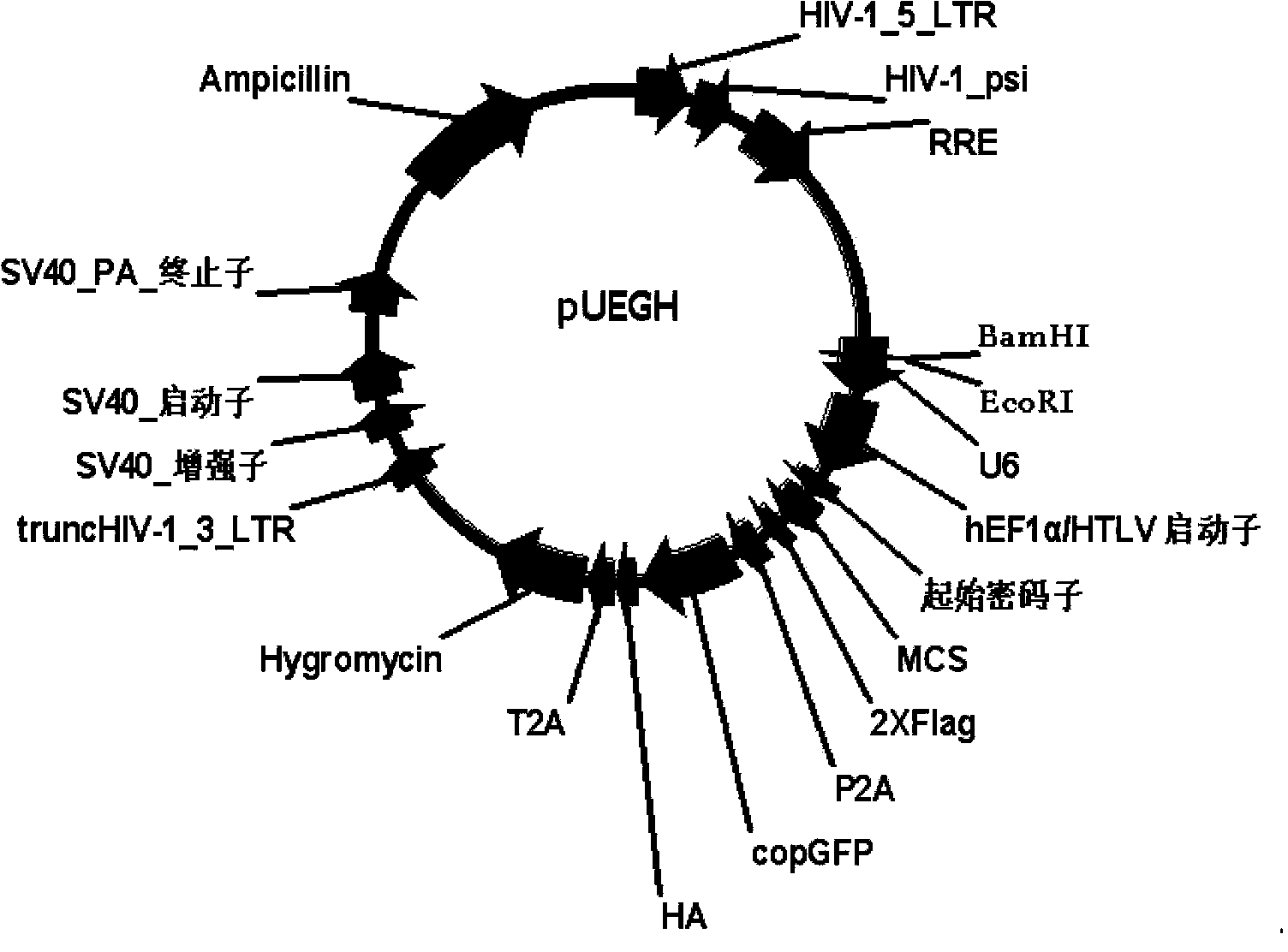
Construction and application of multi-cistron double-label expression lentivirus vector
A technology of lentiviral vectors and recombinant lentiviruses, which is applied in the direction of viruses/bacteriophages, applications, and the use of vectors to introduce foreign genetic materials. and other issues, to achieve the effect of being beneficial to application, good practical value and application prospect, and widely applicable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1U6-E
[0041] Example 1 Construction of U6-EF1α-MCS-FLAG-P2A-Tomato-T2A-Puro (abbreviated as pUETP) lentiviral vector
[0042] (1) Amplify U6, EF1α-MCS-FLAG, Tomato and Puromycin respectively
[0043] 1. Amplification of the U6 promoter
[0044] 1) Whole gene synthesis U6 sequence
[0045] The U6 fragment was artificially synthesized from the whole gene, and its sequence is shown in SEQ ID NO.1.
[0046] 2) Using the whole gene synthesis U6 fragment as a template, the Asc I and Nhe I restriction sites in the U6 fragment were removed by overlap extension PCR, and the EcoR I and BamH I restriction sites were introduced into the sequence at the same time. Wherein, in the first round of PCR, the U6-1 fragment is amplified by U6-F1 and U6-R1 primers, and the U6-2 fragment is amplified by U6-F2 and U6-R2 primers. In the second round of PCR, U6-1 and U6-2 fragments were used as templates, and U6-F1 and U6-R2 were used as primers for PCR amplification to obtain U6 fragments with EcoR Ⅰ an...
Embodiment 2U6-E
[0109] Example 2 Construction of U6-EF1α-MCS-FLAG-P2A-copGFP-T2A-Hygromycin (pUEGH for short) lentiviral vector
[0110] (1) Amplify U6, EF1α-MCS-FLAG, P2A-copGFP and T2A-Hygromycin respectively
[0111] 1. Amplify U6, EF1α-MCS-FLAG
[0112] The method is the same as pUETP.
[0113] 2. Amplification of T2A-Hygromycin
[0114] Using the linear hygro linearized fragment (purchased from Clontech) as a template, the EcoRI cleavage site in Hygromycin was removed by overlapping extension PCR point mutation. Among them, in the first round of PCR, Hygro-1 was amplified using linear hygro as a template, Hygro-F and Hygro-R were used as primers, and Hygro-2 was amplified using Hygro-MF and Hygro-MR as primers. In the second round of PCR, Hygro-1 and Hygro-2 were used as templates, and Hygro-F and Hygro-MR were used as primers for PCR amplification to obtain Hygromycin without an EcoRI restriction site, and T2A was introduced upstream of Hygromycin. Wherein the primer sequence is as ...
Embodiment 3
[0140] Example 3 Application examples of pUETP and pUEGH lentiviral vectors
[0141] (1) Application of pUETP in the construction of CDH1 gene RNAi stable strain
[0142] 1. CDH1 gene shRNA primer design
[0143] According to the principle of shRNA primer design, a pair of shRNA primers were designed for CDH1 gene (NCBI number: NM_004360.3).
[0144] shCDH1-F:
[0145] 5'-GATCCGGCGATTCAAAGTGGGCACAGATGGTACCATCTGTGCC CACTTTGAATCGTTTTTG-3';
[0146] shCDH1-R:
[0147] 5'-AATTCAAAAACGATTCAAAGTGGGCACAGATGGTACCATCTGT GCCCACTTTGAATCGCCG-3'.
[0148] 2. Construction of pUETP-shCDH1 lentiviral vector
[0149] Primer F (10 μM) 10 μl, primer R (10 μM) 10 μl, 10× annealing buffer 5 μl, ddH 2 O25μl reaction system was placed in a 100°C constant temperature water bath for 5 minutes, and then cooled naturally to room temperature for annealing. After the CDH1 shRNA double strand is obtained, it is connected to the EcoRI and BamHI sites of the modified lentiviral vector U6 of the presen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com