Hepatitis B vaccine preparation method using aluminium phosphate adjuvant in-situ method
A technology of hepatitis B vaccine and disodium hydrogen phosphate, which is applied in the field of in-situ preparation of hepatitis B vaccine with aluminum phosphate adjuvant, can solve the problems of increased pain at the injection site of the vaccinators, local redness, increased chance of contamination, cumbersome process operations, etc., and achieves Improving the immune effect and seroconversion rate, reducing the degree of local redness and swelling, and the effect of high antigen adsorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Preparation of Recombinant Hepatitis B Surface Antigen (Hansenula) Stock Solution
[0070] Cell fermentation: Inoculate 200 ml of YNB culture medium with recombinant Hansenula Hepatitis B vaccine working seed batch, and culture at 30°C for 20 hours for primary culture. The primary culture was transplanted into 2L of YNB medium, and cultured at 30°C for 20 hours for secondary culture. The secondary culture was transplanted into 12L of glycerol medium, cultured at 30°C for 88-95 hours, and 24L of Hansenula cell fermentation broth expressing hepatitis B surface antigen was collected.
[0071] Cell disruption: centrifuge the harvested culture at 4°C at 4500 rpm for 20 minutes, remove the supernatant, then add PB buffer to make the cell concentration 30%, and then add 1% of its volume Polysorbate 20 is used to break the cells with a high-pressure homogenizer, so that the breakage rate can reach more than 95%.
[0072] Preliminary purification: add 50% polyethylen...
Embodiment 2
[0078] Embodiment 2 Aluminum hydroxide in situ adsorption method prepares hepatitis B vaccine
[0079] The 5.0L reactor was started to stir at a speed of 200rpm. Add 1000ml of PBS solution, add hepatitis B surface antigen solution (the batch numbers of the antigen stock solution prepared in Example 1 are: B201101, B201102, and the final concentration of antigen is 20 μg / ml), then add 300ml of PBS solution, and then add 10% potassium sulfate dodecahydrate Aluminum solution 250ml, then add 0.85% sodium chloride solution to volume 2500ml. Add 0.5mol / L sodium hydroxide solution to the reaction tank at a flow rate of 50ml / min, and stop adding 0.5mol / L sodium hydroxide solution when the pH is 6.8±0.2. Add 0.85% sodium chloride solution to a volume of 3000ml, continue stirring for 30min, seal the reaction tank, and precipitate.
[0080] The first washing of the precipitate: after 24 hours of precipitation, the supernatant was discarded. Add 0.85% sodium chloride solution to a volu...
Embodiment 3
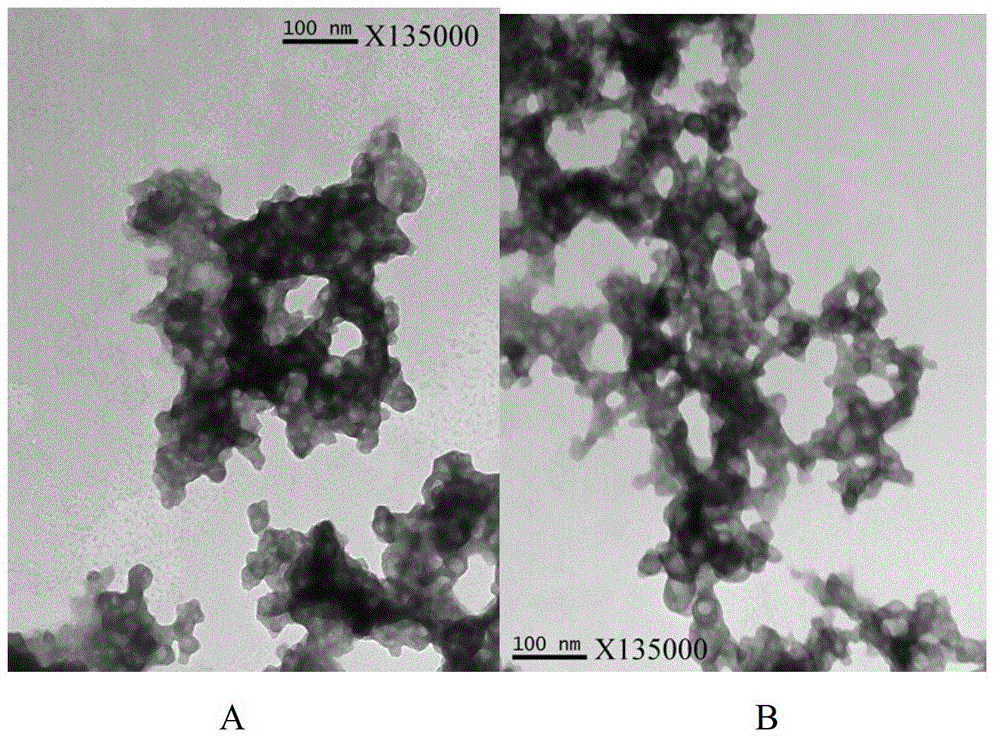
[0084] Embodiment 3 Aluminum phosphate in situ adsorption method prepares hepatitis B vaccine (the present invention)
[0085] Connect the sterile filtration system to the feed port of the 5.0L reactor, pass through the sterile filter, and filter 130.7ml of 10% aluminum chloride hexahydrate solution, 1000ml of sterile water for injection, and 4mol of chlorine at a flow rate of 100ml / min Add sodium chloride solution 51.9ml, hepatitis B surface antigen solution (the batch numbers of the antigen stock solution prepared in Example 1 are: B201101, B201102, B201103, B201104, and the final concentration of antigen is 20μg / ml), 500ml sterile water for injection into the reactor, and turn on Stir at 200rpm. After passing through the sterilizing filter, at a flow rate of 4.0ml / min, add a mixed solution of 5% disodium hydrogen phosphate dodecahydrate solution and 0.5mol / L sodium hydroxide solution to the reaction tank, and add the mixed solution until the pH of the suspension is 3. At 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com