Phosphoryl choline polymer containing aldehyde group and preparation method and application thereof
A technology for phosphorylcholine and polymers, applied in the field of phosphorylcholine polymers and their preparation, capable of solving problems such as complex processes and harsh conditions, and achieving the effects of simple preparation methods, mild conditions, and improved biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of aldehyde-containing phosphorylcholine polymers:
[0033] Weigh methacrolein monomer and 2-methacryloyloxyethyl phosphorylcholine monomer (MPC) in a molar ratio of 3:7, dissolve and mix them in distilled water to obtain a mixed solution of monomers, and mix the total monomers The 1% potassium persulfate of quality dissolves with distilled water to obtain initiator solution, in N 2 Protection, under the condition of stirring at 70°C, add the mixed solution of monomers into the three-necked flask, preheat for 30 minutes, add the initiator solution to continue the reaction for 24 hours, after the reaction is completed, concentrate the reaction solution, and use a dialyzer with a molecular weight cut-off of 6000-8000D Bag dialysis; finally, the dialyzed sample was freeze-dried at -50°C to obtain aldehyde group-containing phosphorylcholine polymer.
[0034] The reaction equation of the present embodiment is as follows:
[0035]
[0036] Preparation of imitat...
Embodiment 2
[0041] The phosphorylcholine polymer containing aldehyde group prepared in Example 1 and phenylenediamine are mixed evenly at 0.5: 1 by aldehyde group and amine group molar ratio, and then coated on the surface of the chitosan material, and then solidified at 20°C After 10 hours of treatment, a coating surface with a membrane structure imitating the outer layer of cells is obtained.
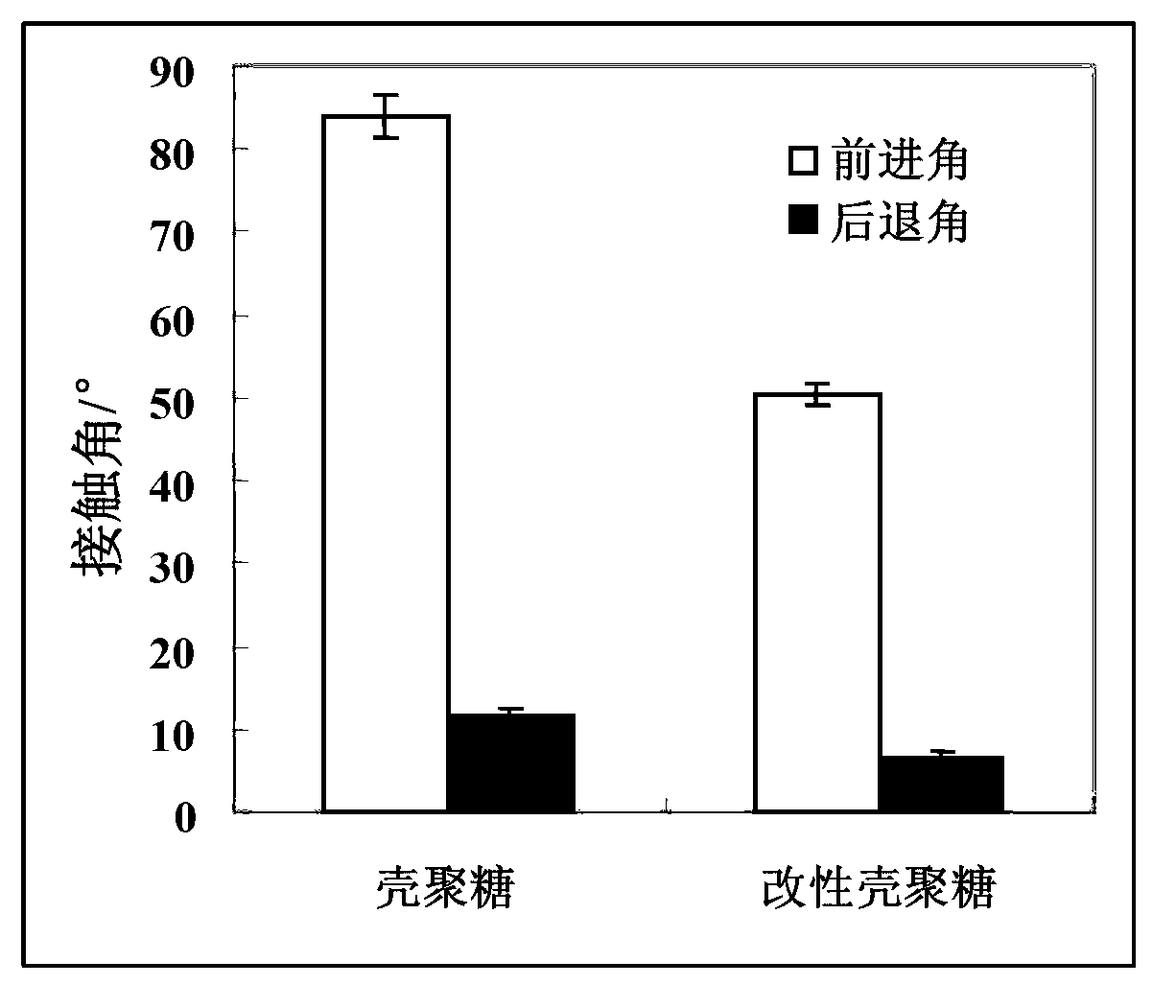
[0042] Compared with the chitosan material without coating treatment, the advancing angle and the receding angle of the chitosan treated by the coating treatment in this embodiment are all reduced, because the hydrophilic Phosphorylcholine polymers with good properties are fixed on the surface of chitosan through the reaction of aldehyde groups and amino groups to obtain a surface with a structure imitating the outer membrane of cells, which significantly improves its hydrophilicity, and its advancing angle and receding angle are obvious. reduce.
Embodiment 3
[0044] The aldehyde group-containing phosphorylcholine polymer prepared in Example 1 and triethylenetetramine are mixed uniformly at 1.2:1 by aldehyde group and amine group molar ratio, and then coated on the surface of the chitosan material, then at 100 ° C After curing for 8 hours, a coating surface with a membrane structure imitating the outer layer of cells was obtained.
[0045] Compared with the chitosan material without coating treatment, the advancing angle and the receding angle of the chitosan treated by the coating treatment in this embodiment are all reduced, because the hydrophilic Phosphorylcholine polymers with good properties are fixed on the surface of chitosan through the reaction of aldehyde groups and amino groups to obtain a surface with a structure imitating the outer membrane of cells, which significantly improves its hydrophilicity, and its advancing angle and receding angle are obvious. reduce.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com