Solid broadband blue-light transmitting organic luminescent material and preparation method thereof
A technology for luminescent materials and fluorescent materials, applied in the field of solid-state broadband blue light emitting organic luminescent materials and their preparation, can solve the problems of less optical materials and the like, and achieve the effects of simple preparation method, strong fluorescence emission performance and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Synthesis of 4,5-dihydro-5-methyl-6-(2,3-diphenyl-6-quinoxalinyl)-3(2H)pyridazinone (compound Ia)
[0030]
[0031] Benzil (i.e. diphenyl ketone, at this time, R in compound II is selected from H, 0.001mol) and 6-(3,4-diaminophenyl)-5-methyl-4,5-di Hydropyridazin-3(2H)-one (Compound III, 0.001mol) was dissolved in 30 ml of glacial acetic acid, stirred and refluxed for 6-8 hours; cooled to room temperature, poured the reaction liquid into ice water under stirring, adjusted with ammonia water pH=7, the obtained solid was filtered with suction, washed with water three times, and the crude product was recrystallized with ethanol-acetone mixed solvent to obtain a white flocculent solid with a yield of 68%. The melting point is 241-243°C.
[0032] 1 H NMR (300MHz, CDCl 3 / TMS)δ:1.36(d,J=7.5Hz,3H),2.57(d,J=16.5Hz,1H),2.83(dd,J=16.9,6.9Hz,1H),3.56-3.61(m,1H ),7.34-7.39(m,6H),7.51-7.56(m,4H),8.18(d,J=9.0Hz,1H),8.39(d,J=2.1Hz,1H),8.41(dd,J= 8.7,2.1Hz,1H),8.91(s...
Embodiment 2
[0034] 4,5-dihydro-5-methyl-6-(2,3-bis(4-methoxyphenyl)-6-quinoxalinyl)-3(2H)pyridazinone (Compound Ib) synthesis
[0035]
[0036] According to the method of Example 1, from anisole (R in compound II is selected from OCH 3 ) and 6-(3,4-diaminophenyl)-5-methyl-4,5-dihydropyridazin-3(2H)-one (compound Ⅲ), the difference is that anisole and compound The molar ratio of Ⅲ is 1:1.1, the solvent is absolute ethanol, the product is light yellow solid, and the yield is 71%. The melting point is 165-167°C.
[0037] 1 H NMR (300MHz, CDCl 3 / TMS)δ:1.35(d,J=7.5Hz,3H),2.56(d,J=16.5Hz,1H),2.82(dd,J=16.9,6.9Hz,1H),3.55-3.62(m,1H ),3.84(s,6H),6.87-6.91(m,4H),7.49-7.53(m,4H),8.12(dd,J=8.4Hz,1H),8.33(s,1H),8.35(dd, J=8.1,2.1Hz,2H),8.98(s,1H); 13 C NMR (75MHz, CDCl 3 / TMS)δ:16.46,27.94,33.90,55.35,113.84,113.91,126.29,127.05,129.36,131.20,131.29,131.35,131.39,135.40,140.87,141.80,152.79,153.64,160.35,160.41,166.66。
Embodiment 3
[0038] Embodiment 3 compound fluorescence performance test
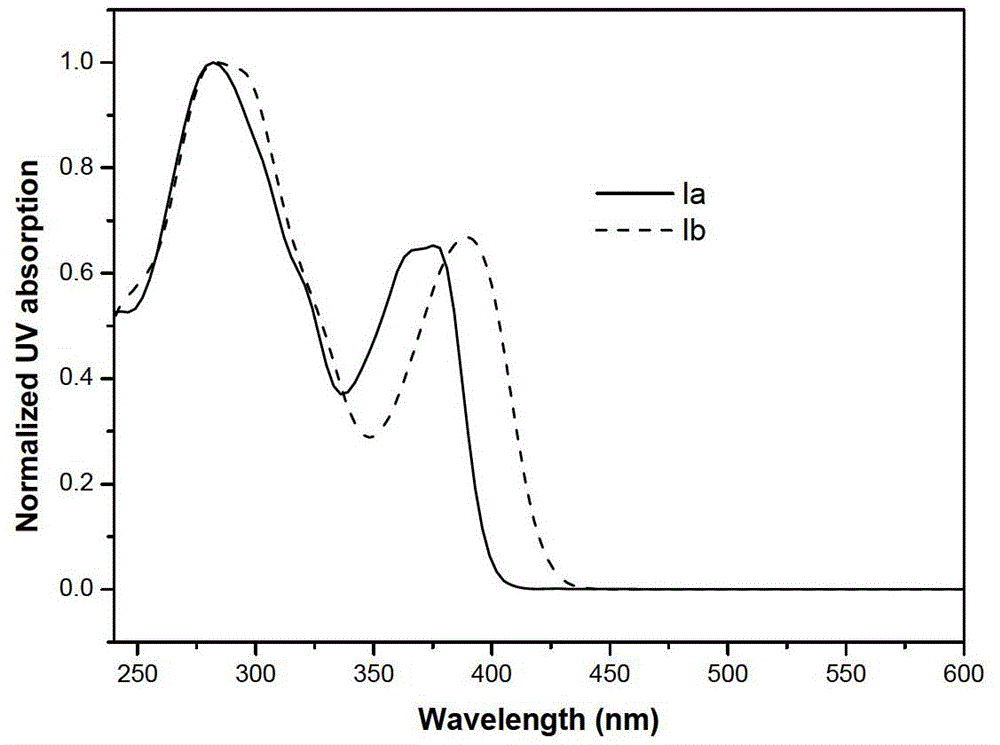
[0039] The compound (Ia, Ib) that embodiment 1 and 2 obtains is prepared respectively and is 2 * 10 -5 M Chloroform solution. Measure the ultraviolet absorption and fluorescence properties on the HORIBA Jobin Yvon Aqualog absorption and three-dimensional fluorescence scanning spectrometer with a 1 cm fluorescence cell, the results are as follows Figure 1-3 shown.
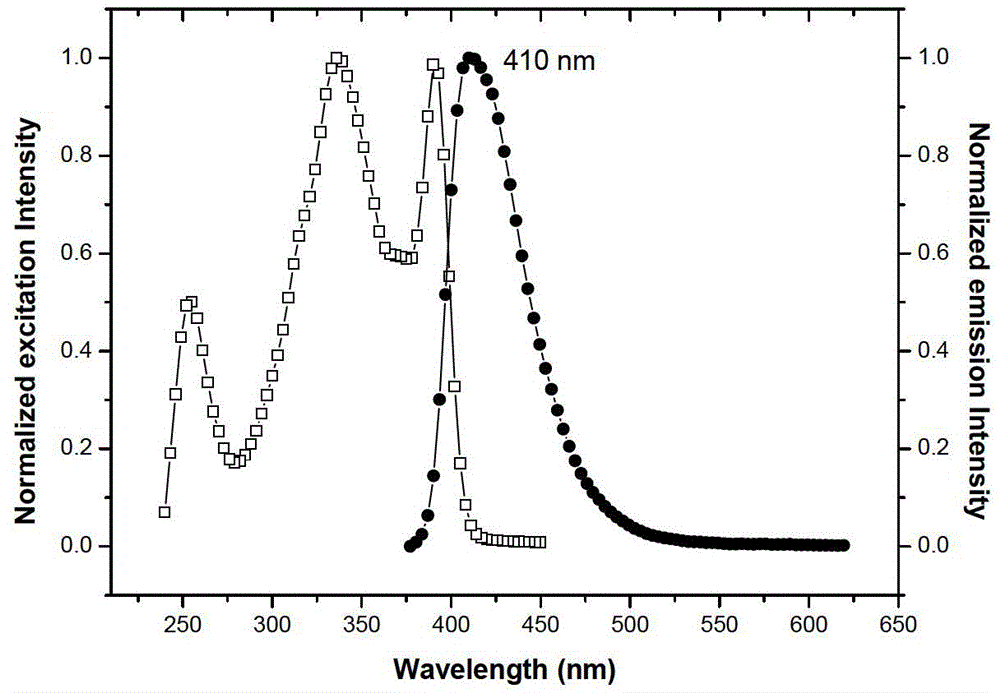
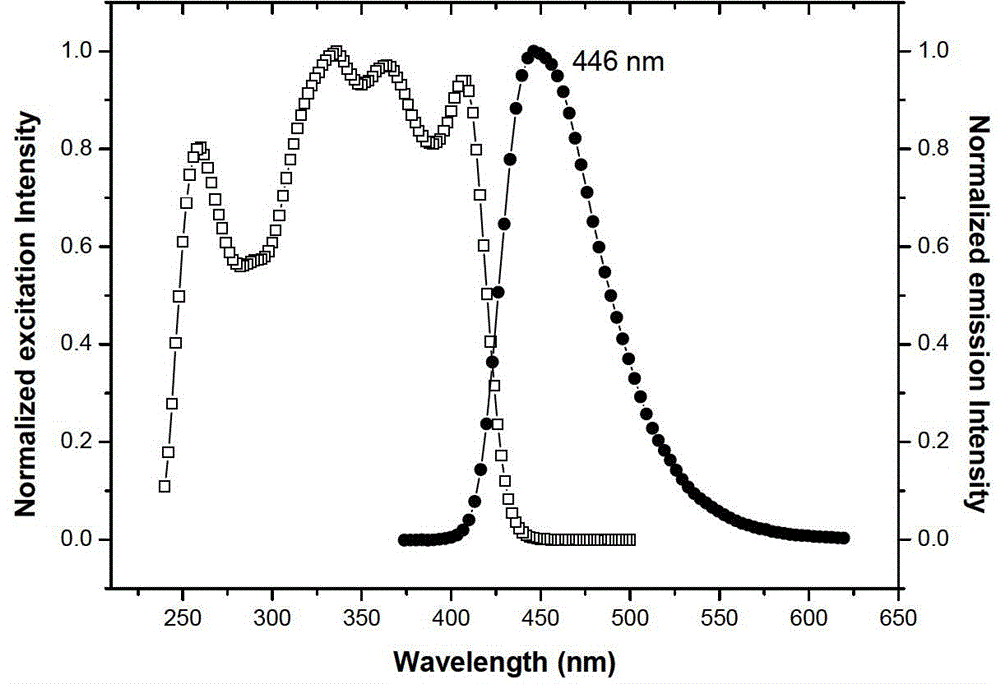
[0040] The solid-state photoluminescence spectrum was measured with a Horiba Jobin-Yvon LabRam HR800 laser Raman spectrometer, and the excitation light source was a 325nm He–Cd laser. The results are shown in Figure 4 .
[0041] Simultaneously observe the fluorescent color of the solid powder of Compound Ia obtained in Example 1 and its chloroform solution under white light and 365nm ultraviolet lamp irradiation, under white light irradiation, Compound Ia is a colorless solution and a white solid respectively in solution and solid state; 365nm ultraviol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com