Method for promoting epidermal cell proliferation
A technology of epidermal cells and epidermal stem cells, applied in applications, animal cells, vertebrate cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
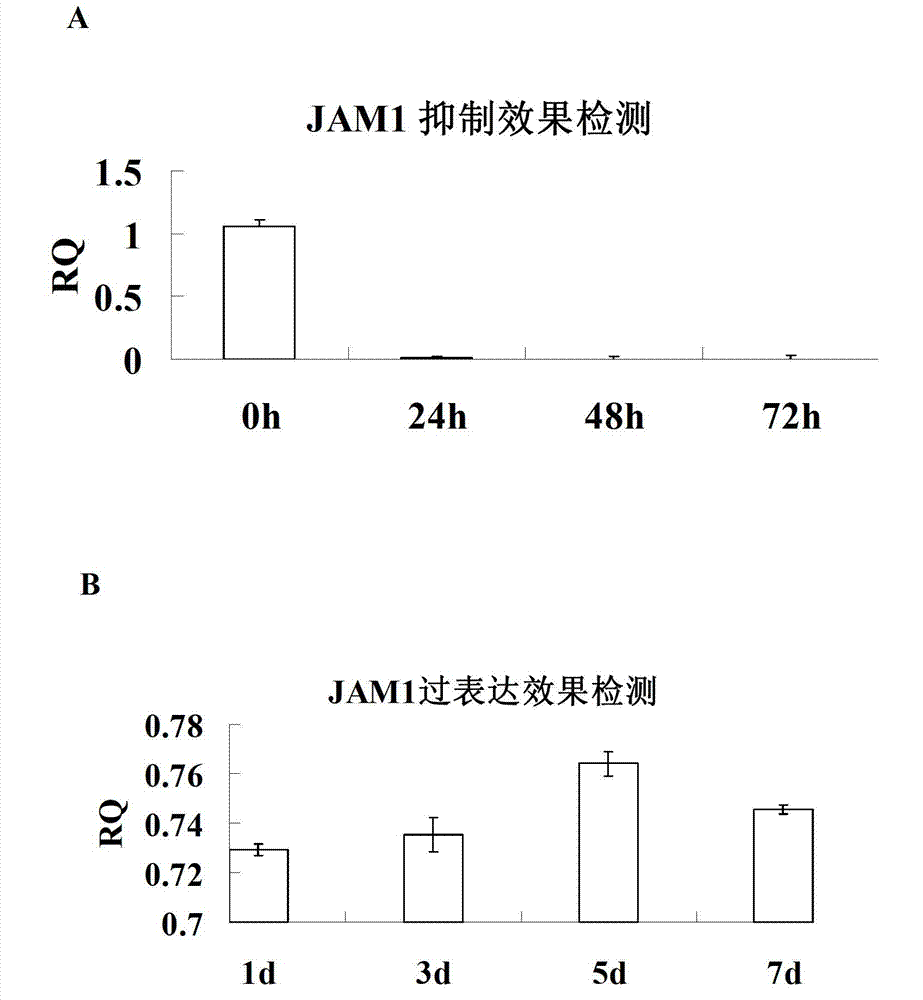
[0077] Example 1: The lentiviral overexpression plasmid pGC-FU-JAM1-GFP was constructed to infect human epidermal cells (hMSC).
[0078] 1. Isolation and culture of epidermal cells
[0079] Adult human skin was obtained from the Plastic Surgery Department of Changhai Hospital. Epidermal cells were obtained from primary culture.
[0080] 2. Preparation of total RNA from human epidermal cells
[0081] The total RNA of human epidermal cells was extracted by the conventional guanidine isothiocyanate method with the total RNA extraction kit of Shanghai Huashun Bioengineering Co., Ltd. Methods as below:
[0082]Take a 3.5cm-diameter dish of epidermal cells growing in a single layer, discard the medium directly, add 1ml of TRIzol to dissolve the cells, and remove the cell lysate with a pipette after the cells are fully dissolved. Incubate the cell lysate sample at 15-30°C for 5 minutes to completely decompose the ribosomes. Add 0.2ml chloroform per 1ml TRIzol, close the cap of t...
Embodiment 2
[0143] Example 2: Cell experiment (in vitro experiment)
[0144] Using fluorescent microscope to take photos, draw growth curves, immunocytochemistry, western-blot and other biological experimental methods, analyze cell morphology changes, target gene expression and cell surface marker keratin expression from various aspects such as cell morphology and protein expression.
[0145] The specific method is as follows:
[0146] 1) Comparison of cell growth status
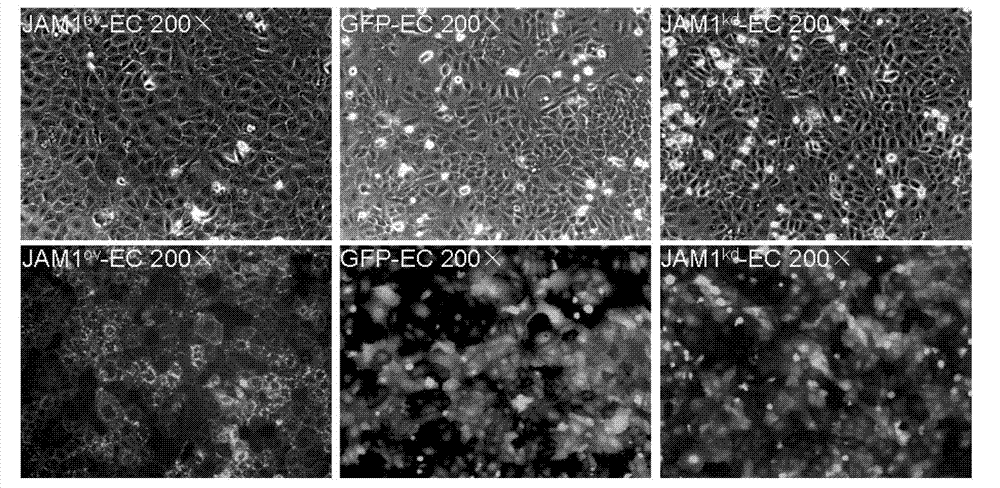
[0147] Visualization of Lentivirally Infected JAM1 Using an Inverted Fluorescence Microscopy ov -EC,JAM1 kd -EC and GFP-EC. Cell morphology did not change significantly, such as figure 1 shown.
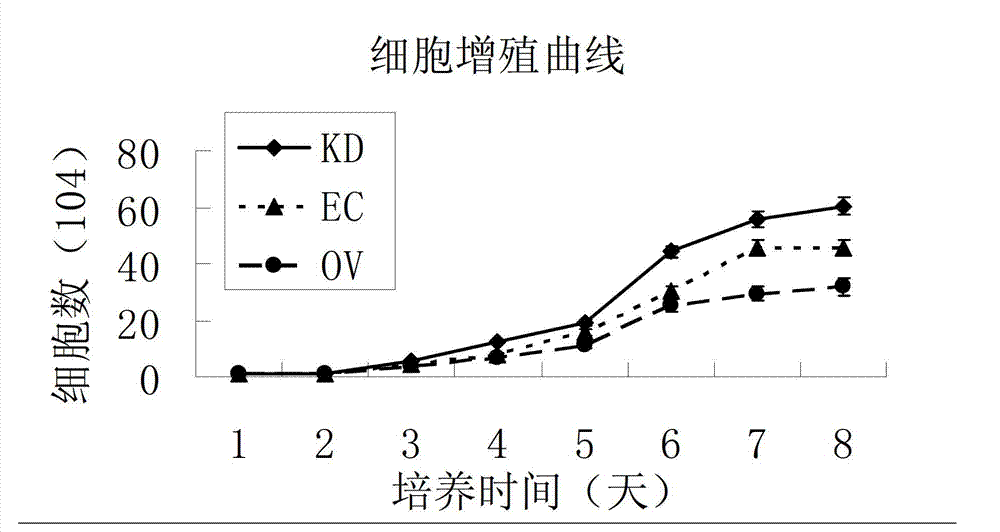
[0148] 2) Draw a growth curve to detect cell proliferation
[0149] JAM1 ov -EC, JAM1 kd -EC and GFP-EC were seeded in 24-well plates with 10,000 cells per well, cultured for 7 days, and digested with trypsin every day to form a single-cell suspension. Cells were replicated in triplicate wells, and the experiment was r...
Embodiment 3
[0191] Example 3: In vivo tumorigenicity test
[0192] Nude mice BALB / c Nu strain, SPF grade, were used in the experiment. Weight about 15 ~ 25g, 3 weeks old, purchased from Shanghai Experimental Animal Center. A total of 12 nude mice were divided into 4 groups: JAM1 ov -EC injection group, GFP-EC injection group, JAM1 kd - EC injection group, PBS injection group. 10 cells 4 0.15ml of the cell suspension was extracted with a 1ml syringe and injected subcutaneously into the back of the forelimb of the nude mouse. They were fed under SPF conditions, observed daily, and samples were collected 4 weeks after transplantation.
[0193] Animals in each group had no difference in appearance, activity, etc. after the experimental treatment. Compared with nude mice in each group after sacrifice, there was no difference in H-E staining, liver, spleen, kidney and other organs.
[0194] H-E staining was done on the skin tissue of the injection site of the nude mice in each group, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com