Production of avian reovirus and vaccine by using mammalian cell line
A technology of poultry reovirus and mammals, applied in the field of veterinary vaccines, can solve the problems of complicated process, high cost and high vaccine price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Using Vero cells to prepare avian reovirus and avian reovirus inactivated vaccine
[0023] (1) Selection of cells for seedling production: African green monkey kidney (Vero) cells were selected and purchased from Shanghai Institute of Biochemistry.
[0024] (2) Passage and culture of cells for seedling production: Vero cells were digested and passaged with EDTA-trypsin cell dispersion solution, and continued to culture at 37°C with cell growth medium. When a good monolayer was formed, they were used for continued passage or virus inoculation.
[0025] (3) Propagation of cytotoxic species:
[0026] Preparation of chicken embryo kidney cells (CK cells): Take 10 17-20-day-old SPF chicken embryos, cut open the abdominal cavity, remove the internal organs, carefully separate the capsule of the kidney with tweezers, then take out the kidney, wash it 3 times with Hank's solution, Then cut it to 1mm 3 Add an appropriate amount of 0.25% trypsin to the cube, and d...
Embodiment 2
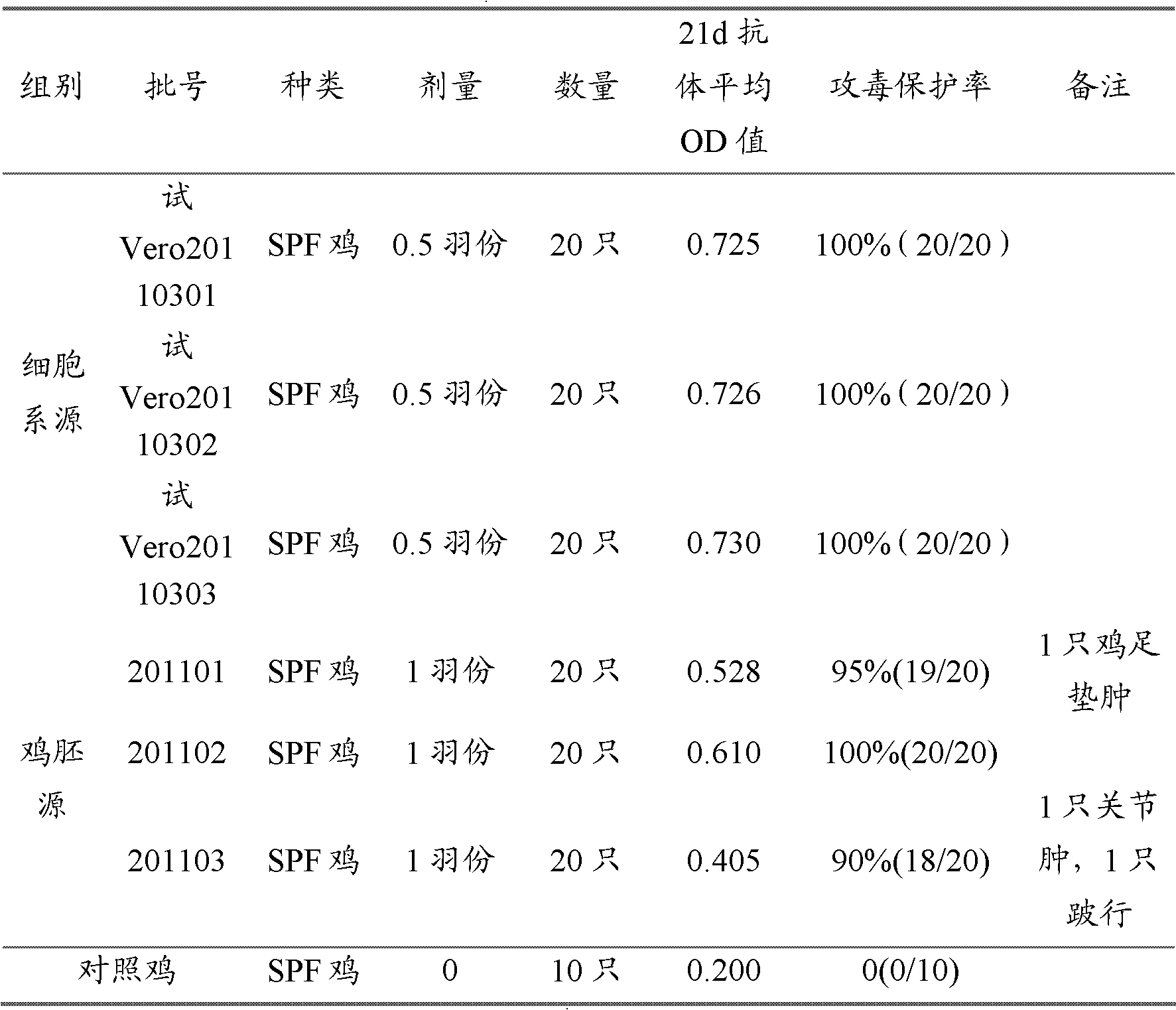
[0036]Embodiment 2: Comparison of test results between cell-derived ARV inactivated vaccine and chicken embryo-derived ARV chicken embryo-derived inactivated vaccine
[0037] 1 material
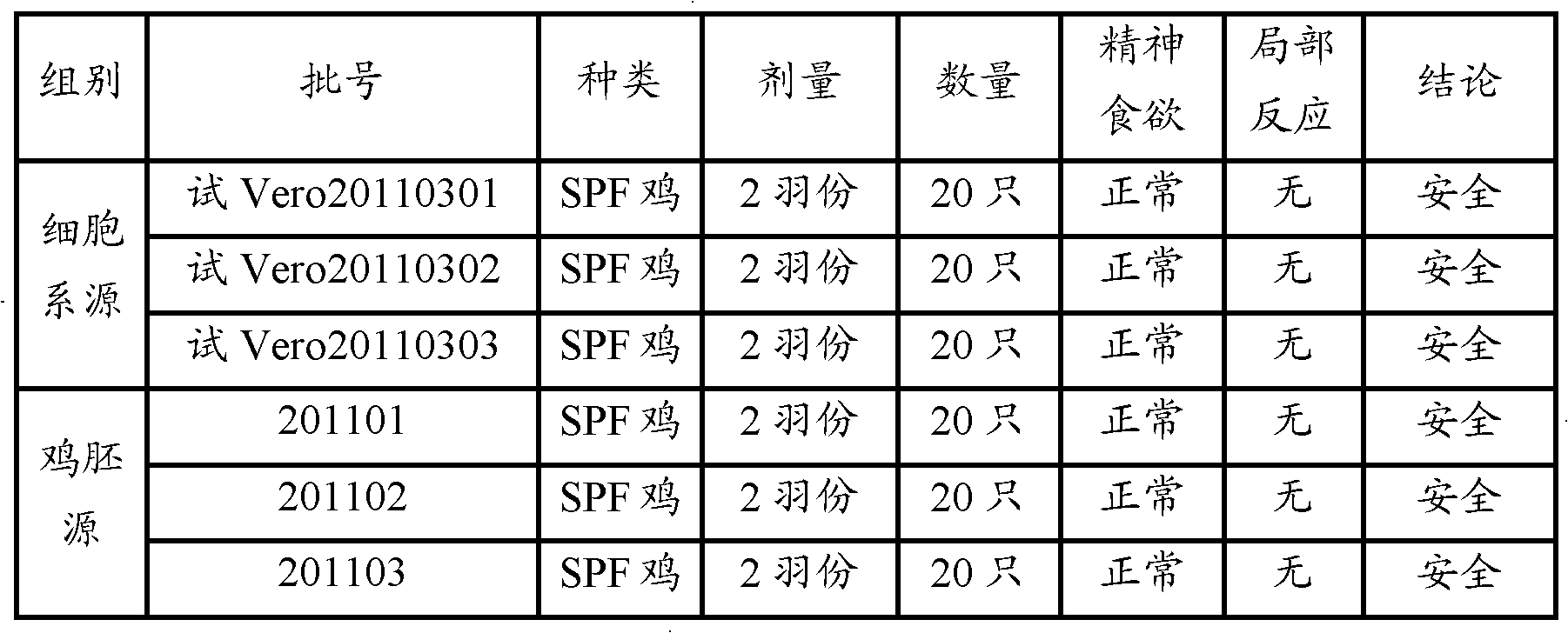
[0038] 1.1 Vaccines Three batches of ARV inactivated vaccines (cell line source) were prepared according to Example 1, batch numbers: Trial Vero20110301, Trial Vero20110302, Vero20110303. There are 3 batches of chicken embryo sources, batch numbers: 201101, 201102, 201103, and the preparation method is shown in 2.1 of this example.
[0039] 1.2 SPF eggs and SPF chickens were purchased from Beijing Meria Weitong Biotechnology Co., Ltd.
[0040] Preparation method and results of 2ARV chicken embryo-derived inactivated vaccine
[0041] 2.1 Preparation of chicken embryo-derived ARV inactivated vaccine:
[0042] a. Virus propagation: AV2311 strain virus seeds are propagated and passed on chicken embryos to make the virus titer detection reach 10 10.5 EID 50 / 0.2ml; b. vaccine inactivation: fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com