Rapid analysis method of copper in leachate
A rapid analysis and leachate technology, applied in material analysis by observing the influence of chemical indicators, analysis by chemical reaction of materials, preparation of test samples, etc., can solve the problems of polluted environment and long time, etc. Achieve the effect of reducing contamination, good accuracy and precision, and shortening analysis and measurement time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
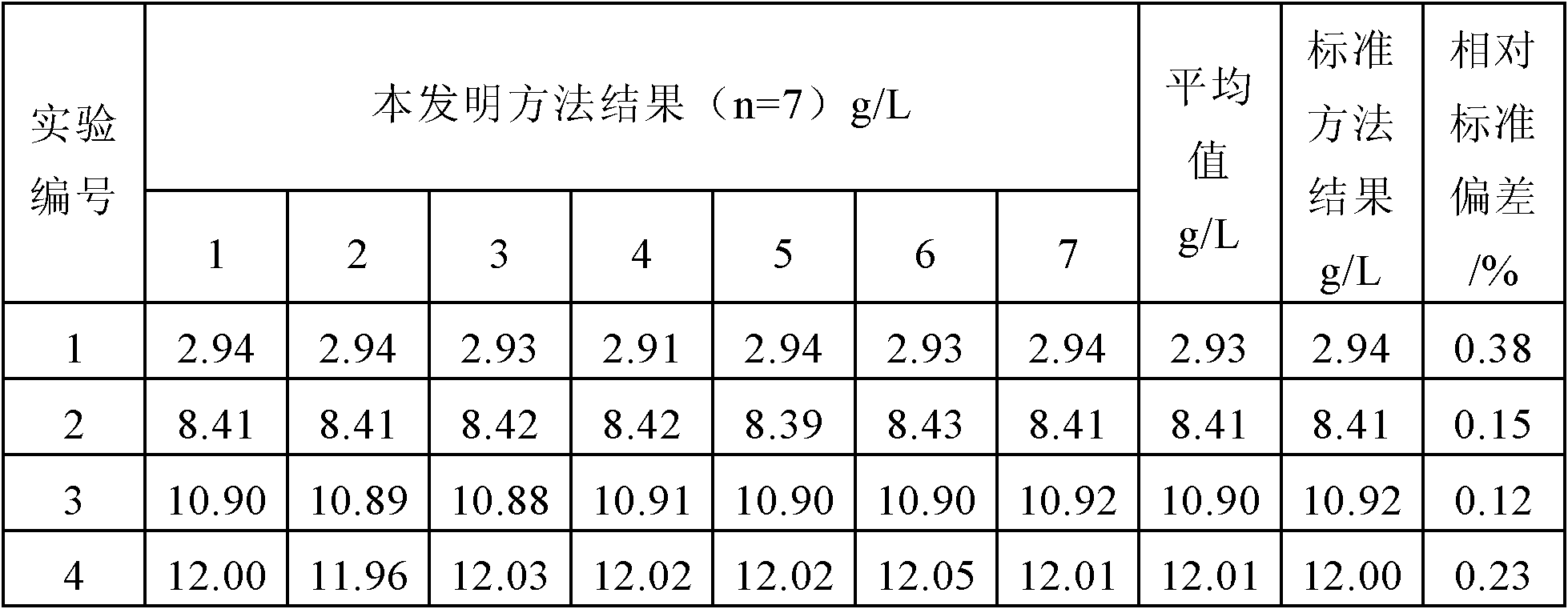
[0018] Pipette 5.00mL of leachate into a 250mL Erlenmeyer flask, add 0.5mL of hydrogen peroxide, heat and evaporate to 1-2mL, cool to room temperature, add acetic acid-ammonium acetate buffer solution (pH≈5) dropwise to the solution until the red color no longer deepens and Excess 2mL, then dropwise add saturated ammonium bifluoride solution until the red color disappears and excess 0.5mL, shake well, add 1-2g potassium iodide to the solution, shake well. Quickly titrate with sodium thiosulfate standard solution (f=1.6333mg / mL) to pale yellow. Add 1mL 5g / L starch solution, continue titrating to light blue, add 1mL 400g / L potassium thiocyanate solution, and titrate again until the blue just disappears, which is the end point. Consumption of sodium thiosulfate standard solution 8.99mL, copper ion concentration in the solution is 2.94g / L.
Embodiment 2
[0020] Pipette 5.00mL of leachate into a 250mL Erlenmeyer flask, add 2.0mL of hydrogen peroxide, heat and evaporate to 1-2mL, cool to room temperature, add acetic acid-ammonium acetate buffer solution (pH≈5) dropwise to the solution until the red color no longer deepens and Excess 2mL, then dropwise add saturated ammonium bifluoride solution until the red color disappears and excess 0.5mL, shake well, add 1-2g potassium iodide to the solution, shake well. Quickly titrate with sodium thiosulfate standard solution (f=1.6333mg / mL) to pale yellow. Add 1mL 5g / L starch solution, continue titrating to light blue, add 1mL 400g / L potassium thiocyanate solution, and titrate again until the blue just disappears, which is the end point. Consumption of sodium thiosulfate standard solution 25.75mL, copper ion concentration in the solution is 8.41g / L.
Embodiment 3
[0022] Pipette 5.00mL of leaching solution into a 250mL Erlenmeyer flask, add 3.0mL of hydrogen peroxide, heat and evaporate to 1-2mL, cool to room temperature, add acetic acid-ammonium acetate buffer solution (pH≈5) dropwise to the solution until the red color no longer deepens and Excessive 2mL, then dropwise add saturated ammonium bifluoride solution until the red color disappears and excess (0.5mL), shake well, add 1~2g potassium iodide to the solution, shake well. Quickly titrate with sodium thiosulfate standard solution (f=1.6333mg / mL) to pale yellow. Add 1mL 5g / L starch solution, continue titrating to light blue, add 1mL 400g / L potassium thiocyanate solution, and titrate again until the blue just disappears, which is the end point. Consumption of sodium thiosulfate standard solution 33.40mL, copper ion concentration in the solution is 10.91g / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com