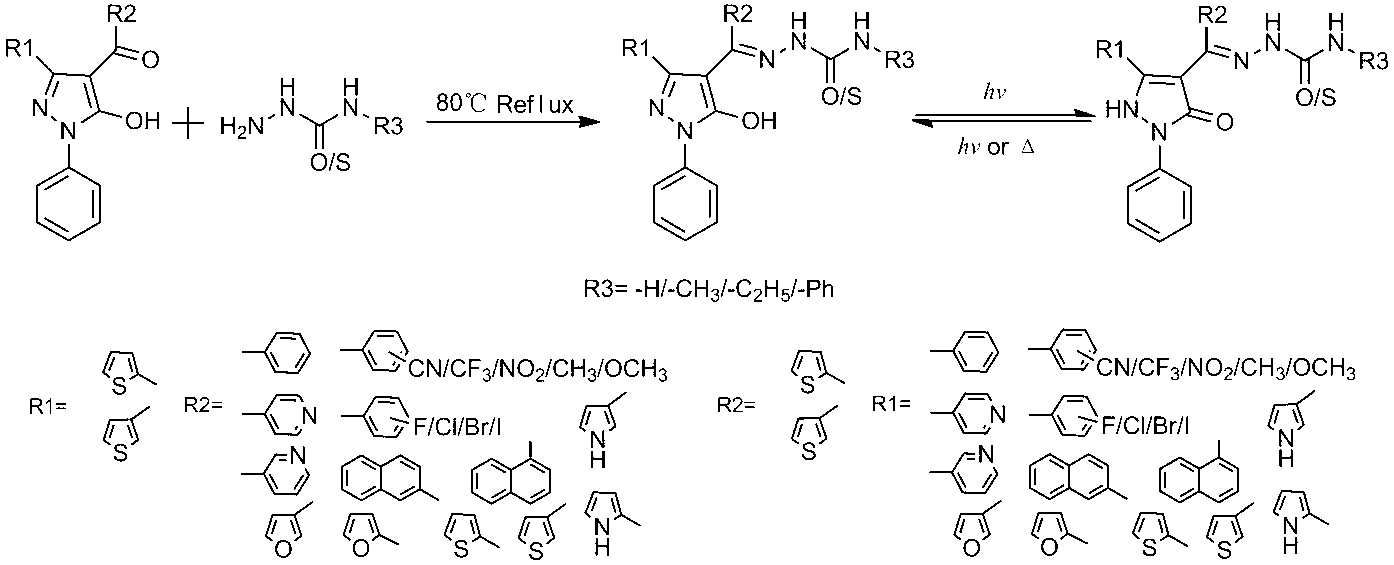
Preparations and applications of thienyl group-containing pyrazolone derivative and its polymer film
A thienyl pyrazolone and polymer film technology is applied in the field of preparation and application of thienyl pyrazolone derivatives and polymer films thereof, and can solve the problems of fatigue resistance, photosensitivity and thermal stability. Practical application, inconvenient promotion and application of solid powder crystals, etc., to achieve the effect of good photochromic performance, simple method and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
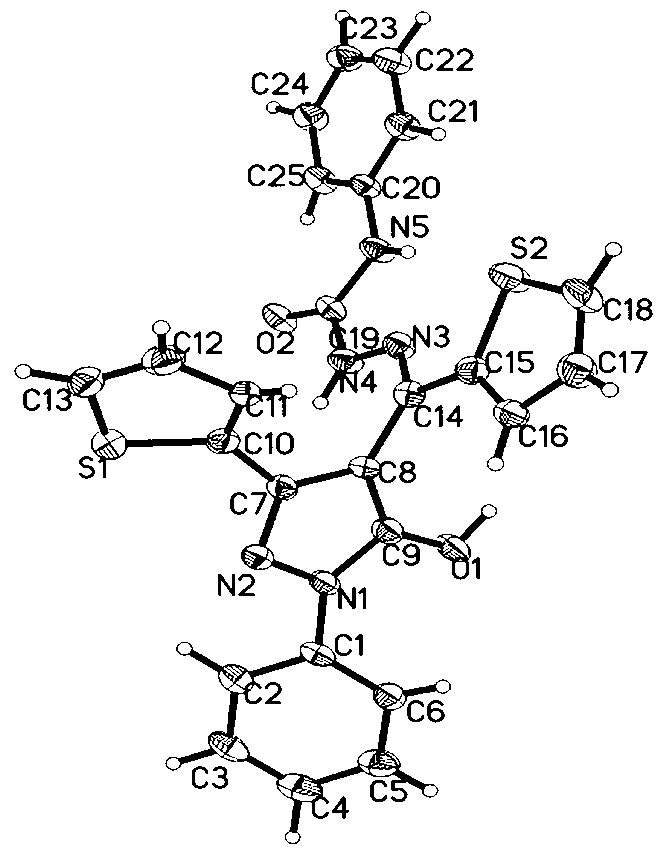
[0017] Embodiment 1: Utilize the preparation method of thienyl pyrazolone derivatives to prepare 1-phenyl-3-thienyl-4-thienyl methylene-5-hydroxypyrazolone phenylsemicarbazide [PD( 2-Th)P-PSC]
[0018] Weigh 1.7627g (5.0mmol) 1-phenyl-3-thienyl-4-thienyl-5-hydroxypyrazolone [PD(2-Th)P-PSC] and 0.7563g (5.0mmol) benzene In a round-bottomed flask, use 5mL of ethanol as solvent and 5-7 drops of glacial acetic acid as catalyst, heat to 80°C with magnetic stirring and reflux for 2h, filter with suction, recrystallize, and dry to obtain 2.1368g of white product (4.4 mmol), yield 88%, melting point: 209.1-210.0°C. The white powder turns yellow after being irradiated with 365nm ultraviolet light; the yellow powder turns from yellow to white after being irradiated with visible light.
[0019] The synthetic route and photochromic mechanism of this type of compound are as follows: figure 1 shown.
Embodiment 2
[0020] Example 2: Preparation of PD(2-Th)P-PSC / PS composite film by method one
[0021] At room temperature, under the condition of magnetic stirring, slowly add 0.25g polystyrene (PS) into 2mL toluene, stir continuously to fully dissolve PS, and form a clear solution. Then, under the condition of avoiding light, slowly add 0.02 g of PD(2-Th)P-PSC white powder into the clear solution, supersede in the ultrasonic instrument for 5min, and continue to stir for 10min to make PD(2-Th)P- The PSC dispersed well into the clear solution, resulting in a co-suspension. After defoaming by standing in the dark, slowly pour the blended suspension into a clean petri dish. The PD(2-Th)P-PSC / PS composite polymer film was obtained by natural air-drying at room temperature in the dark for 24 hours, and the content of PD(2-Th)P-PSC was 8wt%. The film has good photochromic properties.
[0022] The optical photographs of PD(2-Th)P-PSC / PS composite films are as follows figure 2 shown.
Embodiment 3
[0023] Example 3: Preparation of PD(2-Th)P-PSC / PS Composite Film by Method 2
[0024] A common square glass sheet (20mm×20mm×2mm) is used as the substrate, which is cleaned and dried before use. Fix the substrate on a spin coater, drop the blended suspension of PD(2-Th)P-PSC and PS prepared in Example 2 onto the substrate, rotate at a certain speed for 30s, and spin coat The solution was evenly spread into a thin film, and placed at room temperature in the dark to dry naturally to obtain a PD(2-Th)P-PSC / PS composite film with a PD(2-Th)P-PSC content of 8wt%. This film also has photochromic properties.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com