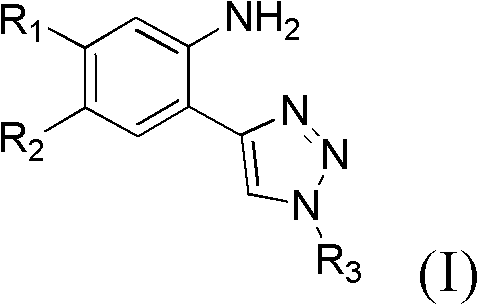
4-ring end substituted 2-1,2,3-triazole phenylamines compound, preparation and purpose
A technology of triazole aniline and compound, applied in the fields of pharmacy, medicinal chemistry and pharmacology, can solve the problems of reversible transaminase rise, rash and vomiting, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The starting materials used in the preparation of the compounds of the present invention are known, can be prepared according to known methods, or are commercially available.
[0041] The invention also relates to new intermediates and / or starting materials. Especially preferred are the same or similar reaction conditions and new intermediates as those mentioned in the examples.
[0042] Both the intermediate and the final product can be post-processed and / or purified according to conventional methods, including pH adjustment, extraction, filtration, drying, concentration, chromatography, grinding, crystallization, and the like.
[0043] In addition, the compounds of the present invention can also be prepared by various methods known in the art or variations of the methods described herein.
[0044] The following examples are only used to illustrate the present invention, and do not limit the present invention in any way.
Embodiment 1
[0045] Example 1, Preparation of 4-ring end substituted 2-1,2,3-triazole aniline compound 2-(1-(3-chloro-4-fluorophenyl)-1H-1,2,3-tri Azazol-4-yl)-5-methoxy-4-(3-morpholinopropoxy)aniline method
[0046]
[0047] It includes the following steps:
[0048] Step a: Preparation of tert-butyl (2-formyl-4-hydroxy-5-methoxyphenyl) aminobenzoate
[0049] 25ml three-necked round bottom flask, add 10ml dichloromethane, compound 2-amino-5-hydroxy-4-methoxybenzaldehyde (0.03g, 0.20mmol), TEA (0.27ml, 0.40mmol), Boc 2 O (0.05g, 0.24mmol), stirred at 0°C for 10 minutes, and then reacted at room temperature for 6 hours. TLC followed the progress of the reaction. After the reaction, use saturated KHSO 4 Adjust the PH value to 7, then extract the triethylamine salt with saturated brine, and the product is in the organic phase. The solvent was removed by rotary evaporation to obtain 0.05 g of the product. (Yield 83.41%). (MS: [M+H] + 268.11)
[0050] Step b: Preparation of tert-butyl(2-formyl-5-me...
Embodiment 2
[0059] Example 2, Preparation of 4-ring-terminally substituted 2-1,2,3-triazole aniline compound 4-(3-(1,3,4-oxothioazin-4-yl)propoxy)-2 -(1-(3-chloro-4-fluorophenyl)-1H-1,2,3-triazol-4-yl)-5-methoxyaniline method
[0060]
[0061] The preparation method is as shown in Example 1. The raw material in step b is 4-(3-chloropropyl)-1,3,4-oxythioazine, and the remaining steps are the same, and the final product is 0.07g.
[0062] MS: [M+H] + 480.12; 1 H NMR(400MHz, CDCl 3 ): δppm 7.79(s, 1H), 7.48(d, 1H), 7.33(d, 1H), 7.18(d, 1H), 7.00(s, 1H), 6.27(s, 1H), 6.13(s, 1H) ), 4.64(s, 2H), 4.06(t, 2H), 4.05(s, 1H), 3.83(s, 1H), 3.49(t, 2H), 2.72(t, 2H), 2.55(t, 2H) , 1.84 (m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com