Compound preparation for treating porcine contagious pleuropneumonia and respiratory tract mixed infection and preparation method thereof
A compound preparation, the technology of pleuropneumonia, is applied in the directions of anti-inflammatory agents, respiratory diseases, non-central analgesics, etc., which can solve problems such as difficulty in controlling the disease and curing, economic losses for farmers, and untimely treatment, and achieve blood medicine. The effect of long-term concentration maintenance, strong drug penetration and strong killing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
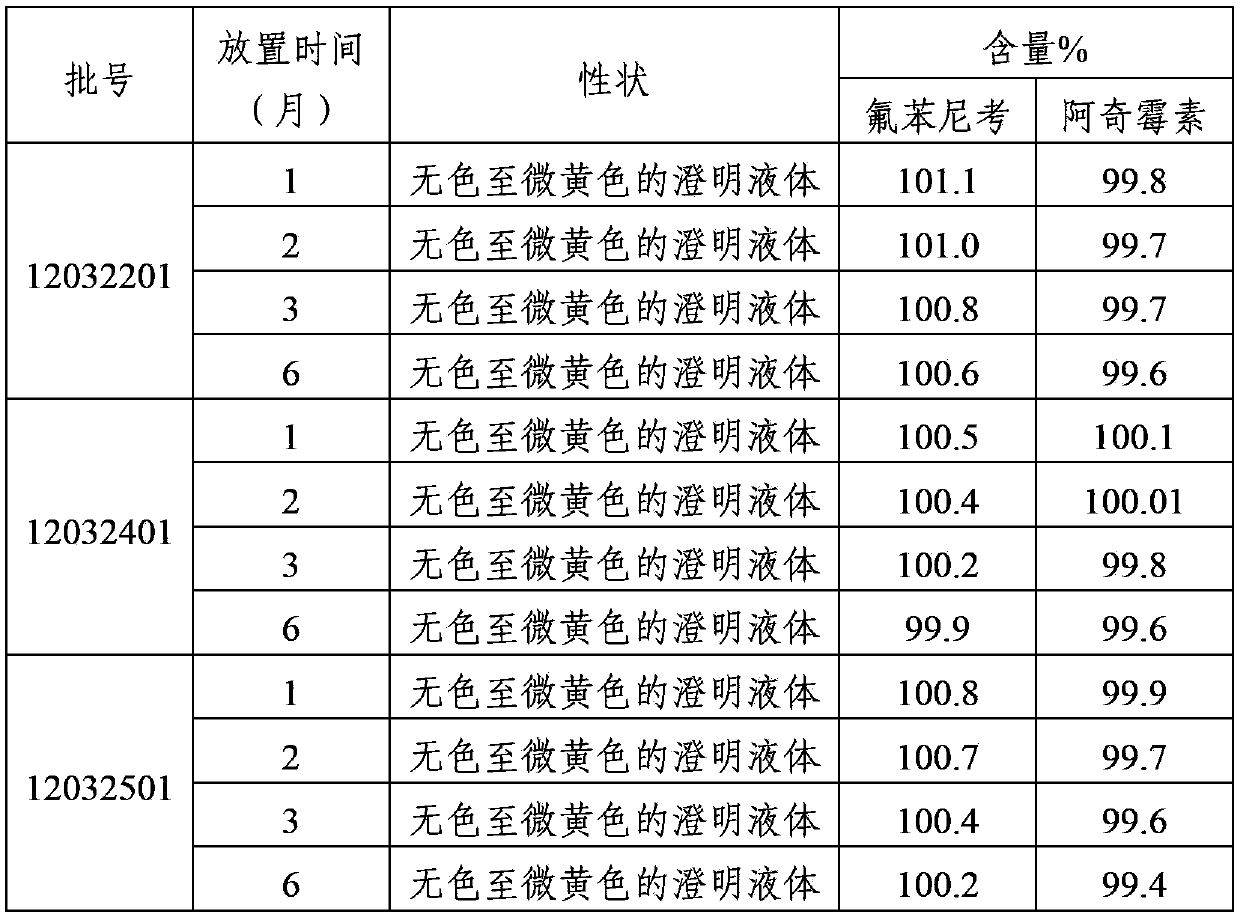
Image
Examples
Embodiment 1
[0030] The compound preparation of the present embodiment comprises the following components: florfenicol 10g, azithromycin 5g, diclofenac sodium 2.5g, trimethoprim 3g, dimethylformamide 30g, polyvinylpyrrolidone 1g, a- Add 14 g of pyrrolidone, 20 g of propylene glycol, 0.04 g of dexamethasone, and absolute ethanol to 100 ml.
[0031] Described preparation method comprises the steps:
[0032] (1) Mix 20g of dimethylformamide and 10g of propylene glycol, add polyvinylpyrrolidone and a-pyrrolidone, heat to 50°C, add florfenicol, azithromycin, and sodium diclofenac in sequence, and stir until dissolved to obtain A liquid;
[0033] (2) Take 10g of dimethylformamide, 10g of propylene glycol and 10g of absolute ethanol to form a composite solvent, heat to 80°C, add trimethoprim and stir until dissolved to obtain liquid B;
[0034] (3) Combine liquid A and liquid B, add dexamethasone pre-dissolved with 3ml of absolute ethanol, add absolute ethanol to 100ml, filter, fill with nitrog...
Embodiment 2
[0036] The compound preparation of this embodiment comprises the following components: Florfenicol 20g, azithromycin 10g, diclofenac sodium 5g, trimethoprim 6g, dimethylformamide 30g, polyvinylpyrrolidone 2g, α-pyrrolidone 13g, 20g of propylene glycol, 0.08g of dexamethasone, and absolute ethanol were added to 100ml.
[0037] Described preparation method comprises the steps:
[0038] (1) Mix 20g of dimethylformamide and 10g of propylene glycol, add polyvinylpyrrolidone and a-pyrrolidone, heat to 55°C, add florfenicol, azithromycin, and sodium diclofenac in sequence, and stir until dissolved to obtain A liquid;
[0039] (2) Take 10g of dimethylformamide, 10g of propylene glycol and 10g of absolute ethanol to form a composite solvent, heat to 70°C, add trimethoprim and stir until dissolved to obtain liquid B;
[0040] (3) Combine liquid A and liquid B, add dexamethasone pre-dissolved with 4ml of absolute ethanol, add absolute ethanol to 100ml, filter, fill with nitrogen, and s...
Embodiment 3
[0042] The compound preparation of this embodiment contains the following components: Florfenicol 10g, azithromycin 5g, aminopyrine 4g, trimethoprim 3g, dimethylformamide 30g, polyvinylpyrrolidone 1g, N-methyl- 15g of 2-pyrrolidone, 20g of propylene glycol, 0.04g of dexamethasone, and add absolute ethanol to 100ml.
[0043] Described preparation method comprises the steps:
[0044] (1) Mix 20g of dimethylformamide and 10g of propylene glycol, add polyvinylpyrrolidone and N-methyl-2-pyrrolidone, heat to 60°C, add florfenicol, azithromycin, and aminopyrine in turn, and stir until Dissolved to obtain liquid A;
[0045] (2) Take 10g of dimethylformamide, 10g of propylene glycol and 10g of absolute ethanol to form a composite solvent, heat to 60°C, add trimethoprim and stir until dissolved to obtain liquid B;
[0046] (3) Combine liquid A and liquid B, add dexamethasone pre-dissolved with 2ml of absolute ethanol, add absolute ethanol to 100ml, filter, fill with nitrogen, and ster...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com