Linear alkyl benzene synthesis method
A technology of straight-chain alkylbenzene and synthesis method, which is applied in the addition of unsaturated hydrocarbons and saturated hydrocarbons to hydrocarbon production, organic chemistry, and bulk chemical production, etc. It can achieve the effect of high olefin conversion rate, avoiding frequent switching operations of device reaction and regeneration, and good activity stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Solid superacid Ni / SO4 2- -SnO 2 Catalyst preparation.
[0020] A certain mass of SnCl 4 ·5H 2 O was dissolved in deionized water to prepare a solution with a mass fraction of 5%, and slowly added dropwise ammonia water with a mass fraction of 28% under rapid stirring to adjust the pH value of the solution to 8, and left to stand at room temperature for aging overnight. After suction filtration, the filter cake was subjected to 4% CH 3 COONH 4 The solution is washed until the pH value is equal to 7, and dried at 110°C to obtain the precursor Sn(OH) 4 ; Grind the precursor to a particle size of less than 110 mesh powder. Ni(NO3) 2 ·6H 2 O was dissolved in 3.0 mol / L sulfuric acid solution to prepare a sulfuric acid solution of nickel nitrate with a concentration of 0.5 mol / L. According to the ratio of liquid to solid ratio of 15mL / g, take 2g of Sn(OH) 4 , immersed in the above solution under stirring, so that Ni 2+ and SO4 2- Loaded on Sn(OH) 4 above, dried a...
Embodiment 2
[0022] Using solid super acid Ni / SO4 2- -SnO 2 The catalyst carries out the alkylation reaction of benzene and olefins.
[0023] The alkylation raw materials used are industrial benzene and industrial alkene mixed hydrocarbons (C10-C13), wherein the industrial alkene mixed hydrocarbons have a linear olefin content of 10.3% and a diene mass content of 0.4%.
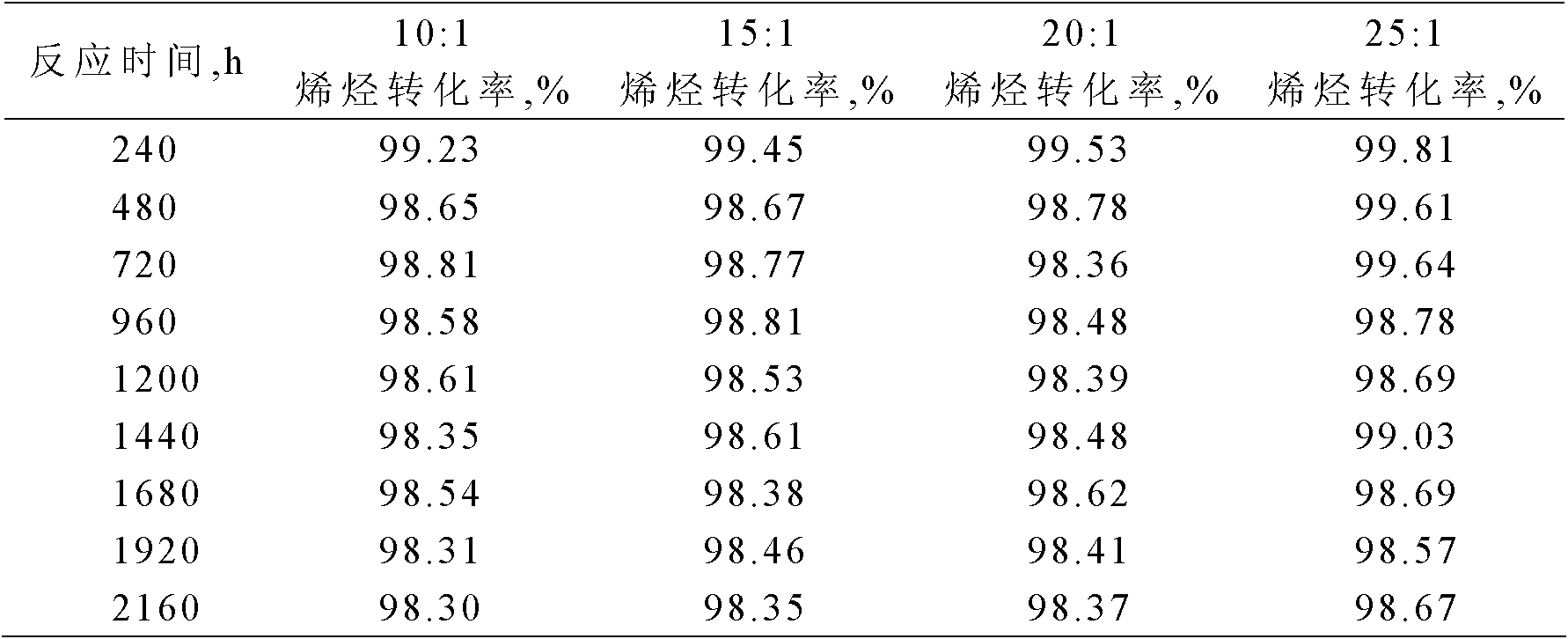
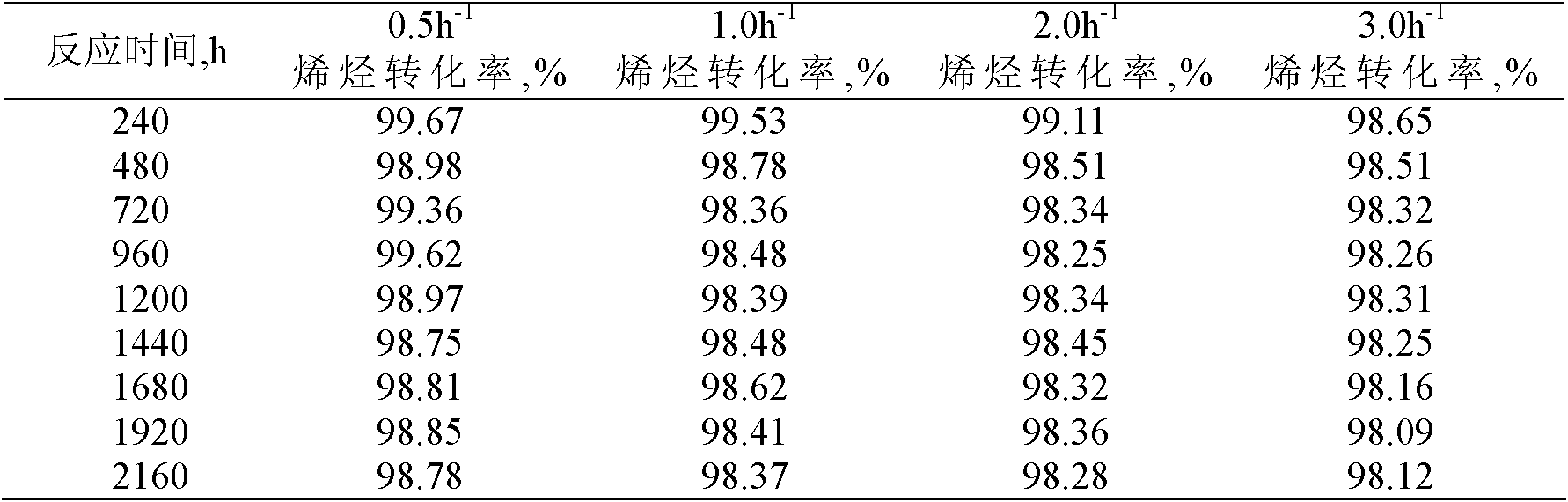
[0024] Using a fixed-bed reaction device, 4.0 grams of the catalyst with a particle size of less than 110 mesh prepared in Example 1 was loaded into the reactor. After the catalyst was loaded into the reactor, it was first purged with nitrogen (60ml / min) at 160°C for 2 hours for catalyst activation treatment, then the reactor temperature was adjusted to 150°C and the pressure was adjusted to 3.0MPa. The molar ratio of substances is 20:1, and the mass space velocity is 1.0h -1 The alkylation reaction is carried out continuously under the conditions, and samples are taken regularly to measure the conversion rate of olefin...
Embodiment 3
[0028] The alkylation reaction between benzene and olefins was carried out under different temperature conditions.
[0029] The alkylation raw materials used are industrial benzene and industrial alkene mixed hydrocarbons (C10-C13), wherein the industrial alkene mixed hydrocarbons have a linear olefin content of 10.3% and a diene mass content of 0.4%.
[0030] Using a fixed-bed reaction device, 4.0 grams of the catalyst with a particle size of less than 110 mesh prepared in Example 1 was loaded into the reactor. After the catalyst was loaded into the reactor, it was first purged with nitrogen (60 ml / min) at 160° C. for 2 hours to carry out catalyst activation treatment. Then, at a pressure of 3.0 MPa, the ratio of benzene to olefins in the feed is 20:1, and the mass space velocity is 1.0 h -1 Continuous alkylation reactions at different temperatures were carried out under the same conditions, samples were taken regularly, and the olefin conversion rates at different reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com