Tanshinone IIA-polyactic acid/hydroxyacetic acid microsphere and preparation method thereof
A technology of glycolic acid microspheres and glycolic acid, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low bioavailability, affecting clinical efficacy, and low solubility, etc. problem, to achieve the effect of reducing microvessel density, inhibiting tumor growth, and high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
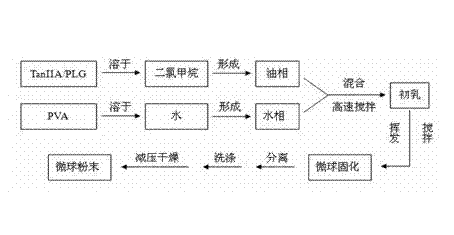
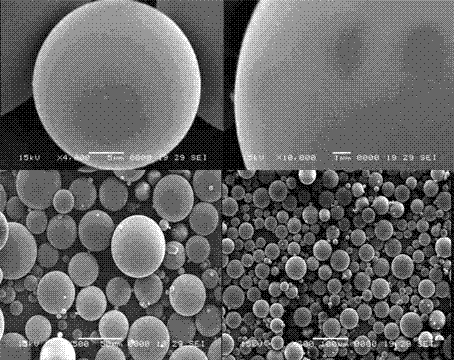
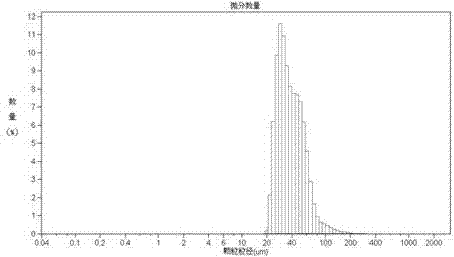
[0052] The oil phase (dispersed phase) is PLGA with a weight to volume ratio of 30% (g / ml) 75 / 25 - Dichloromethane solution, the water phase (continuous phase) is a PVA-water solution with a weight volume ratio of 0.5% (g / ml), and the oil phase / water phase ratio is 1:10 (volume ratio). The drug loading, that is, the content of tanshinone ⅡA is 3.5% by weight. Homogenize under the condition of 2900rpm×3min. After forming colostrum, pour it into 400ml of PVA-water solution with a weight-volume ratio of 0.5%. Stirring speed is 200rpm, Dichloromethane was volatilized at 35°C to solidify the microspheres. After sieving, the PVA attached to the surface was washed off with 400 ml of pure water, and dried under reduced pressure to obtain microsphere powder. Flowchart such as figure 1 shown.
[0053] Among them, Tanshinone Ⅱ A was purchased from Xi'an Guanyu Biotechnology Company, with a purity of ≥98%. PLGA: monomer ratio lactic acid / glycolic acid = 75 / 25, Mw 15kD, ester-capped, pu...
Embodiment 2
[0055] The oil phase (dispersed phase) is PLGA with a weight to volume ratio of 10% (g / ml) 70 / 30 -Dichloromethane solution, the water phase (continuous phase) is a PVA-water solution with a weight-to-volume ratio of 1% (g / ml), and the oil phase / water phase ratio is 1:10 (volume ratio). The drug loading, that is, the content of tanshinone ⅡA is 1% by weight, homogenized under the condition of 2900rpm×3min, and poured into 200ml of PVA-water solution with a weight-volume ratio of 1% after forming colostrum, at a stirring speed of 200rpm, Dichloromethane was volatilized at 35°C to solidify the microspheres. After sieving, the PVA attached to the surface was washed off with 400 ml of pure water, and dried under reduced pressure to obtain microsphere powder.
[0056] Among them, Tanshinone Ⅱ A was purchased from Xi'an Guanyu Biotechnology Company, with a purity of ≥98%. PLGA: monomer ratio lactic acid / glycolic acid=70 / 30, ester-terminated, purchased from Shandong Institute of Medi...
Embodiment 3
[0058] The oil phase (dispersed phase) is PLGA with a weight to volume ratio of 20% (g / ml) 85 / 15-Dichloromethane solution, the water phase (continuous phase) is PVA-water solution with a weight volume ratio of 0.6% (g / ml), and the oil phase / water phase ratio is 1:10 (volume ratio). The drug loading, that is, the content of tanshinone ⅡA is 6% by weight, homogenized under the condition of 2900rpm×3min, and poured into 300ml of PVA-water solution with a weight-volume ratio of 0.6% after forming colostrum, at a stirring speed of 200rpm, Dichloromethane was volatilized at 35°C to solidify the microspheres. After sieving, the PVA attached to the surface was washed off with 400 ml of pure water, and dried under reduced pressure to obtain microsphere powder.
[0059] Among them, Tanshinone Ⅱ A was purchased from Xi'an Guanyu Biotechnology Company, with a purity of ≥98%. PLGA: monomer ratio lactic acid / glycolic acid = 85 / 15, ester-terminated, purchased from Shandong Institute of Medi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com