Colloidal gold partly modified by polyethylene glycol and preparation method thereof
A polyethylene glycol and colloidal gold technology, applied in the field of analytical chemistry, can solve problems affecting the accuracy of analysis results, colloidal gold agglomeration, false positives, etc., to solve uncontrollable agglomeration and sedimentation, good dispersion performance, and enhanced stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
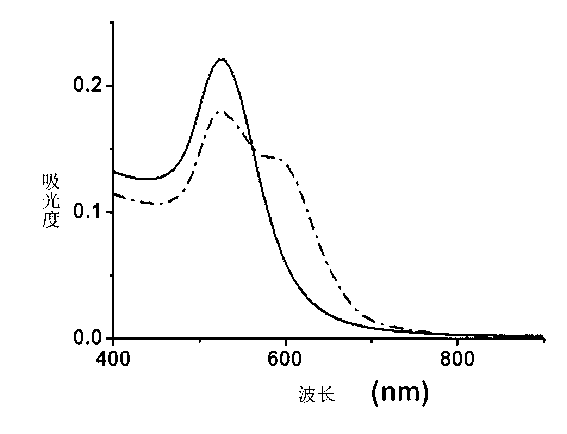
[0033] The following is the colloidal gold with an average particle diameter of 43 nanometers partially modified by applying the method of the present invention to prepare polyethylene glycol 2-thioethyl ether acetic acid (Poly(ethylene glycol) 2-thioethyl ether acetic acid, average molecular weight 1000), and using The colloidal gold detects mercury ions (Hg 2+ ) specific examples of content:
[0034]First, immerse the coverslip (20 mm×20 mm) in a beaker containing 10 ml of a mixed solution of 98% concentrated sulfuric acid and 30% hydrogen peroxide (volume ratio 4:1) for 5 minutes, and then put the beaker into the sonicator Sonicate in a water bath for 15 minutes; then take out the treated coverslip, wash it three times with secondary water, immerse it in a solution containing 0.05 M dodecyltrimethylammonium bromide, and let it stand overnight; The coverslip assembled with dodecyltrimethylammonium bromide was taken out, rinsed three times with secondary water, and immersed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com