ABA type amphiphilic triblock copolymer based on molecular glue and uses of the same
A copolymer and amphiphilic technology, which is applied in the field of ABA-type amphiphilic triblock copolymers, can solve the problems of time-consuming and energy-consuming, complicated purification process and high cost, and achieves good stability and simple and easy synthesis of raw materials. the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
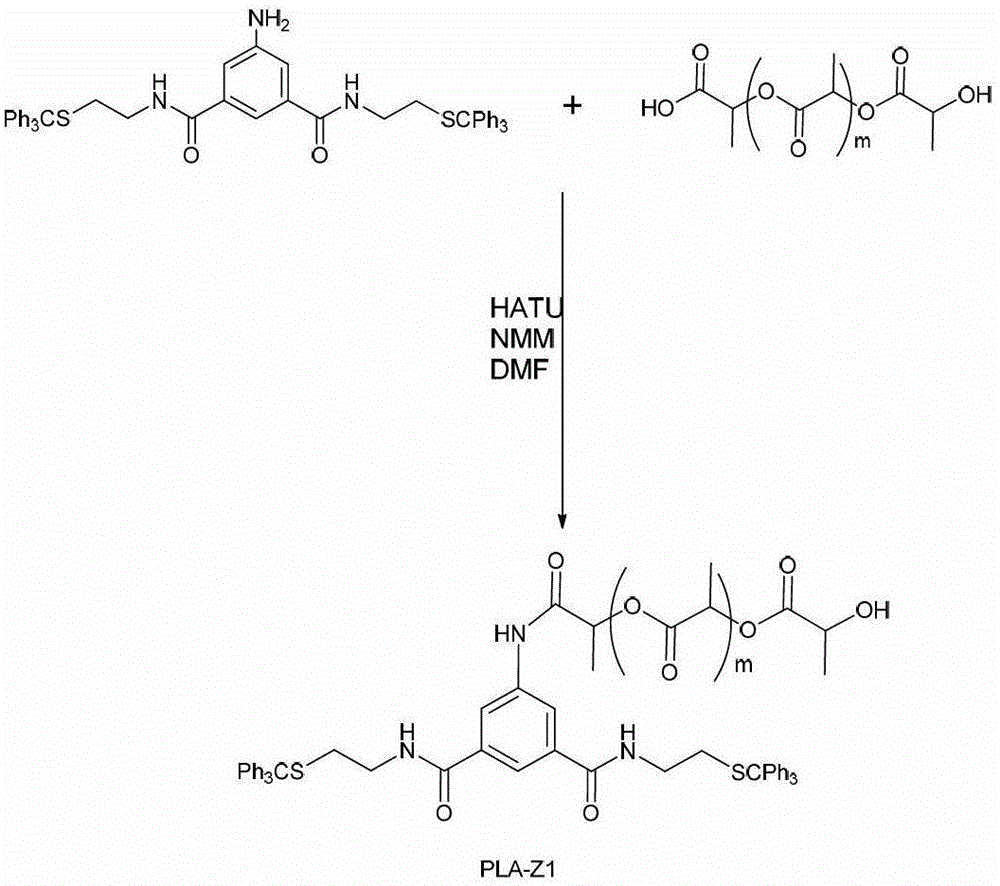
[0051] Embodiment 1, the synthesis of amphiphilic triblock copolymer PEG-PLA-PEG and PLA-PEG-PLA
[0052] 1. Synthesis of Hydrophilic Fragment PEG-A4
[0053] (1) When n=2 of PEG, Mn=163;
[0054] Its synthetic route is as follows figure 1 Shown: Under alkaline conditions, compound A4 is subjected to amidation reaction with PEG163 whose terminal is an amino group to obtain compound PEG163-A4;
[0055] The steps are specifically: weigh A4 (0.841g, 1mmol) and dissolve 15ml DMF in a 25ml single-necked flask, add NMM (N-methylmorpholine) (112 μL, 1.0mmol) under stirring in an ice-water bath, HATU (2 -(7-azobenzotriazole)-N, N, N', N'-tetramethyluronium hexafluorophosphate) (0.57g, 1.5mmol) after activation for 30min (TLC monitors that the activation reaction is complete), Add PEG163 (0.163g, 1mmol) with an amino group at the end, stir for 1h, then warm up to room temperature and react for 10h, stop the reaction (TLC monitors the end of the reaction), add an appropriate amount...
Embodiment 2
[0134] Embodiment 2, the mensuration of block copolymer molecular weight
[0135] Adopt GPC method to measure the molecular weight of the polymer prepared in Example 1; Instrument: Agilent1260 type gel permeation chromatography, GPC column: 7.5 * 300mm, 10 μm gel chromatography column, solvent: tetrahydrofuran, flow rate: 1.0mL / min , column temperature: 35°C, standard sample: polystyrene. The GPC molecular weight distribution diagram of each polymer is as follows Figure 7 shown.
Embodiment 3
[0136] Embodiment 3, preparation and characterization of micelles (taking PEG5000-PLA5000-PEG5000 as an implementation example)
[0137] 1. Preparation of blank micelles
[0138] Accurately weigh 10 mg of the synthesized block copolymer, dissolve it in 2 mL of DMF (also can be DMSO or THF), slowly drop the block copolymer DMF solution into 10 ml of stirred PBS buffer solution (also can be pure water or normal saline ) (10min / ml), stirred for 30min to form microemulsion balls, transferred to a dialysis bag, dialyzed with PBS buffer for 48 hours, changed the water once every 4h, and dialyzed DMF to obtain self-assembled micelles.
[0139] 2. Preparation of drug-loaded micelles
[0140] Accurately weigh 10 mg of the synthesized block copolymer and 1 mg of DOX.HCI to dissolve in 2 mL of DMF (also DMSO or THF), add 20 μl of triethylamine and stir for 30 min. Or camptothecin; what adopted in the present embodiment is azithromycin) the synthesized block copolymer DMF solution slo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com