Synthetic method of methoxamine hydrochloride
A technology of methoxamine hydrochloride and its synthesis method, which is applied in the field of chemistry, can solve problems such as difficult industrial production, high requirements for synthesis conditions, unstable oximation products, etc., and achieve short preparation cycle, high purity, and less environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
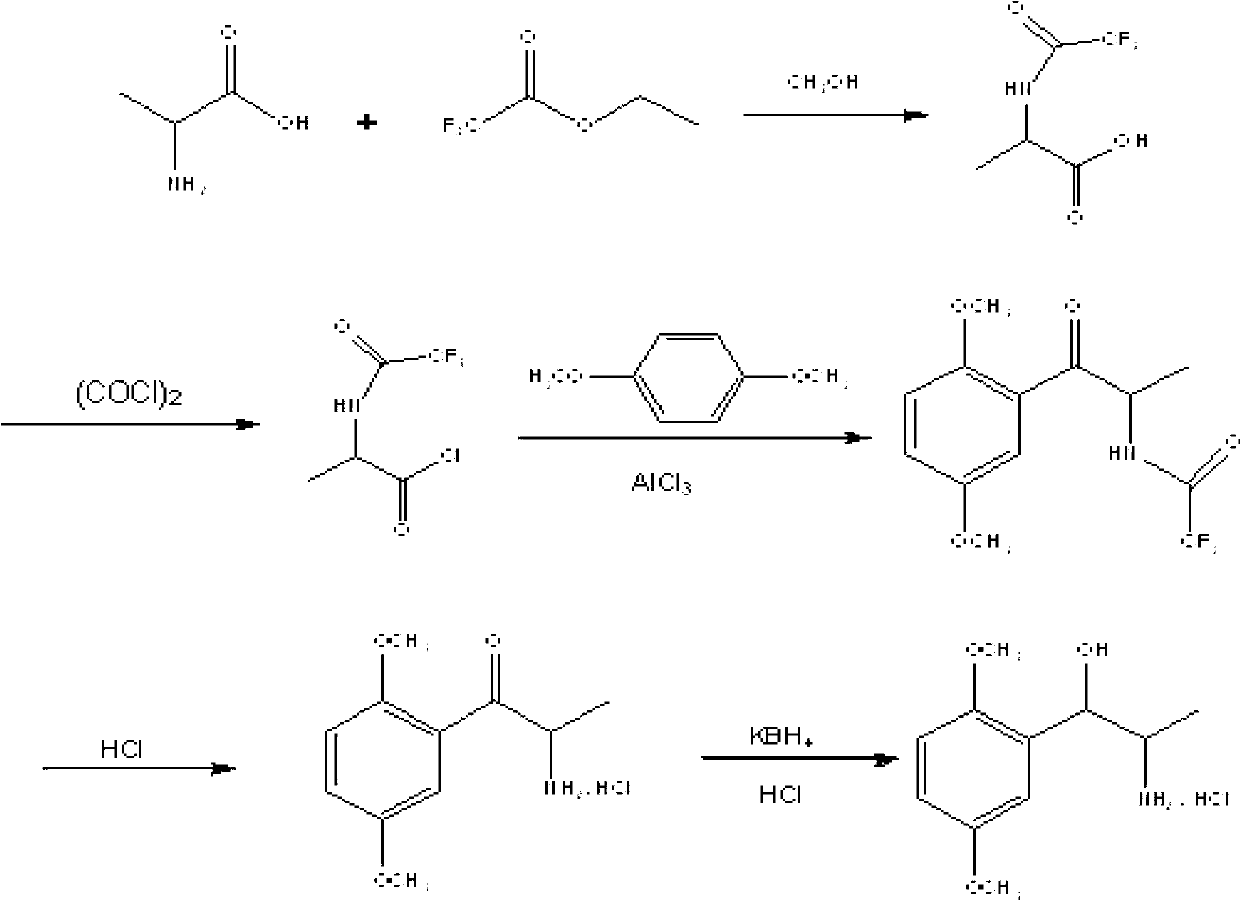
[0036] Embodiment 1, the preparation of N-trifluoroacetyl-DL-α-alanine
[0037] Add 100g of DL-α-alanine and 500ml of anhydrous methanol to a 1000ml three-necked reaction flask, stir at room temperature, add 160ml of triethylamine dropwise at room temperature, add 160g of ethyl trifluoroacetate dropwise, control the temperature at 25°C, and stir the reaction 8h, during the reaction process, the solution gradually turned into a light yellow clear liquid, and the HPLC monitored the completion of the reaction; the methanol solvent was evaporated under reduced pressure, and a white solid appeared, which was dissolved in 500ml purified water, and the pH value was adjusted to 1 with 36% (g / g) concentrated hydrochloric acid. -2 or so, extracted three times with ethyl acetate, 200ml each time, combined the organic phases, washed with 200ml purified water, separated into layers, kept the organic layer, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressur...
Embodiment 2
[0038] Example 2, Preparation of 2-trifluoroacetyl-1-(2,5-dimethoxyphenyl)-1-propanone
[0039] Add 185g of N-trifluoroacetyl-DL-α-alanine alanine and 500ml of dichloromethane to a 1000ml reaction bottle, stir and cool down to 15°C, add 121g of oxalyl chloride dropwise, and control the temperature at 15°C; 1.5h After dropping, react for 1 hour; rise to room temperature, react for 3 hours, weigh 118g of p-xylylene dimethyl ether and dissolve in 200ml of dichloromethane, drop the mixture into the reaction bottle, drop it within 20 minutes, react for 1 hour at 15°C, and react for 8 hours at room temperature. After the reaction is complete, pour the reaction solution into 500ml of ice water, stir while adding, separate the liquids, wash the organic phase twice with water, and dry with anhydrous sodium sulfate; After drying, 213g of Intermediate II was obtained, with a yield of 83.1%, and HPLC of 99.3%.
Embodiment 3
[0040] Example 3, 2-amino-1-(2,5-dimethoxyphenyl)-1-propanone hydrochloride
[0041] Add 180 g of 2-trifluoroacetyl-1-(2,5-dimethoxyphenyl)-1-propanone, 108 g of 36% (g / g) concentrated hydrochloric acid, and 250 ml of ethanol into a 1000 ml single-port reaction flask, and heat to Reflux, the reaction solution is gradually light yellow and clear, reflux reaction for 4h, the reaction is completed, the reaction solution is concentrated under reduced pressure at 70°C, and the liquid is evaporated to dryness to obtain a reddish-brown oily liquid. Add 100ml of n-hexane and 100ml of dichloromethane to the residue, and wash for 2h , filtered and dried to obtain 136 g of light yellow solid, yield 82.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com