Copper-hydroxyapatite catalyst for synthesizing methyl glycolate and ethylene glycol and preparation method thereof
A kind of technology of methyl glycolate and hydroxyapatite, applied in the field of chemical industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
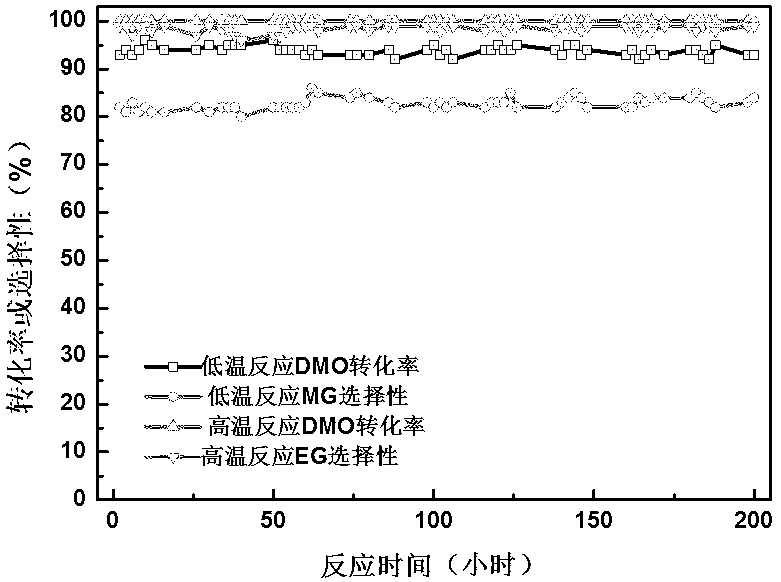
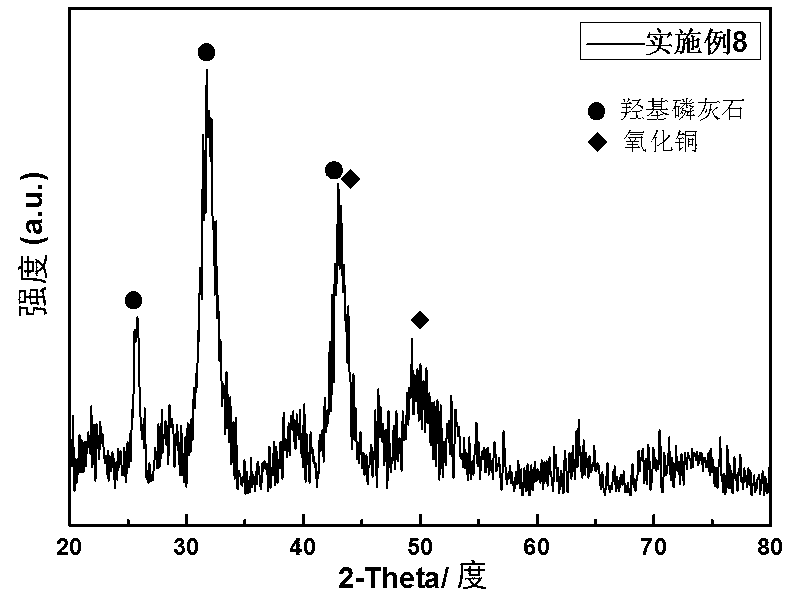
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of carrier hydroxyapatite
[0021] 23.52 g Ca(NO 3 ) 2 4H 2 O was dissolved in 300 mL water, and the pH was adjusted to 11~12 with ammonia water to make a calcium nitrate solution (about 0.33 M), and 200 mL containing 7.89 g (NH 4 ) 2 HPO 4 The aqueous solution (about 0.30 M, Ca / P=1.67 (mol / mol)) was slowly dropped into the above calcium nitrate solution, and stirred and heated at 50°C for 16 h to obtain a suspension containing apatite carrier for use. The mass fraction of hydroxyapatite in the suspension is about 2%.
[0022] Active metal copper and auxiliary metal loading: 13.10 g Cu(NO 3 ) 2 ·3H 2 O and 3.01 g Fe(NO 3 ) 3 9H 2 Add O to the carrier suspension at the same time, adjust the pH of the suspension to 11~12 with ammonia water, stir and react in a water bath at 30°C for 14 hours, then increase the temperature of the water bath, and continue stirring at 80°C until the suspension The pH value is 9~10, the reaction is stopped, ...
Embodiment 2
[0024] Example 2: Preparation of carrier hydroxyapatite
[0025] 23.52 g Ca(NO 3 ) 2 4H 2 O was dissolved in 300 mL of water, and the pH was adjusted to 11~12 with ammonia water to make a calcium nitrate solution (about 0.33 M), and 200 mL containing 4.39 g (NH 4 ) 2 HPO 4 The aqueous solution (about 0.20 M, Ca / P=3.0 (mol / mol)) was slowly dropped into the above calcium nitrate solution, and stirred and heated at 50°C for 16 h to obtain a suspension containing apatite carrier for use. The mass fraction of hydroxyapatite in the suspension is about 2%.
[0026] Active metal copper and auxiliary metal loading: 13.10 g Cu(NO 3 ) 2 ·3H 2 O and 7.56 g Mn(NO 3 ) 2 4H 2 Add O to the suspension containing the carrier hydroxyapatite at the same time, adjust the pH of the suspension to 10~11 with ammonia water, stir and react in a water bath at 50 °C for 6 h, increase the temperature of the water bath, and continue to stir and react at 80 °C , until the pH value of the suspens...
Embodiment 3
[0028] Embodiment 3: The preparation of carrier hydroxyapatite is the same as that in Embodiment 1.
[0029] Active metal copper and auxiliary metal loading: 13.10 g Cu(NO 3 ) 2 ·3H 2 O and 1.91 g Zn(NO 3 ) 2 ·6H 2 Add O to the suspension containing the carrier hydroxyapatite at the same time, adjust the pH of the suspension to 11~12 with ammonia water, stir and react in a water bath at 50°C for 6 h, increase the temperature of the water bath, and continue to stir and react at 80°C , until the pH value of the suspension is 7~7.5, stop the reaction, obtain the suspension containing the catalyst, then filter it, wash it with deionized water for 5 times, bake the filter cake at 120°C, and put it in a muffle furnace for 10 °C / min, the temperature was raised to 600 °C and kept for 5 h to obtain catalyst powder.
[0030] Activity evaluation is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com