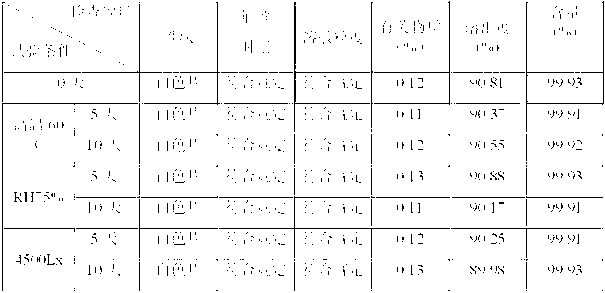Lelrozol orally-disintegrating tablet and its preparation method
A technology for oral disintegrating tablets and letrozole, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and pill delivery, etc., can solve the problems of affecting the therapeutic effect, difficult and difficult dissolution of letrozole, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
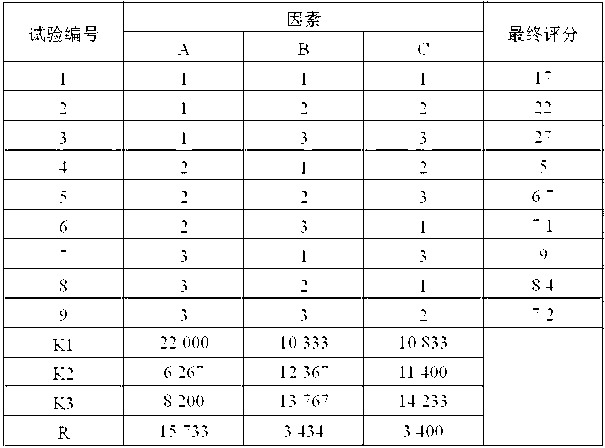
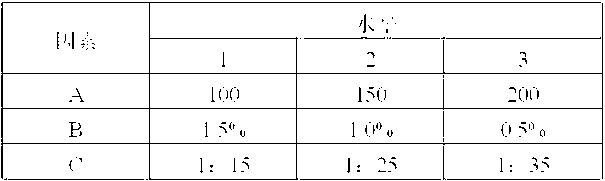
Image
Examples
Embodiment 1
[0051] Weight ratio of raw and auxiliary materials:
[0052] Letrozole 250g, polyethylene glycol 6000 8750g, maltodextrin 1000g, hydrolyzed gelatin 20g.
[0053] Preparation Process:
[0054] (a) Preparation of dispersion: Weigh polyethylene glycol 6000 and letrozole in proportion, heat and melt polyethylene glycol 6000 and add letrozole, stir well, fully cool, and pulverize through a 100-mesh sieve to obtain Dorozole dispersion is ready for use;
[0055] (b) Ingredients: dissolving the hydrolyzed gelatin into a 0.5% aqueous solution, adding the letrozole dispersion and maltodextrin obtained in step (a) in proportion, and fully stirring to obtain a mixed material;
[0056] (c) Freeze-drying: put the mixed material obtained in step (b) into a suitable mold, and after pre-freezing at -40°C for 12 hours, enter the sublimation stage, the vacuum degree is 1.03mba, and the partition temperature is 5°C; in the analysis stage, the vacuum degree 0.77mba, the partition temperature is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com