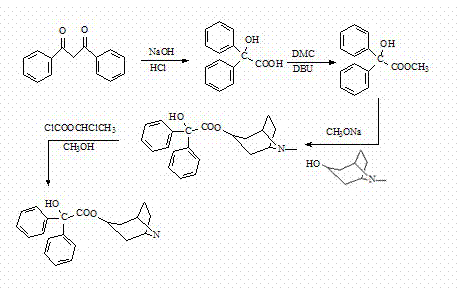
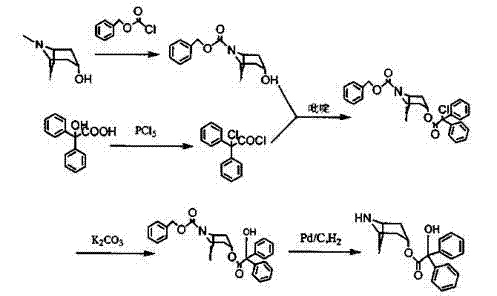
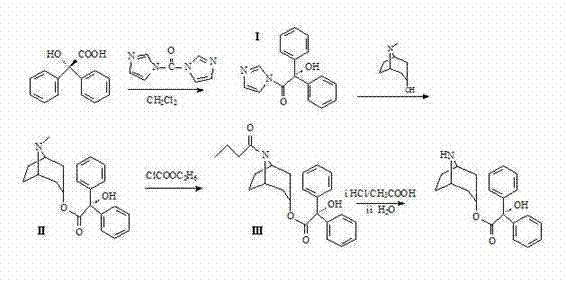
Method for preparing 2-hydroxy-2,2-diphenylacetic acid-3alpha-(8-aza-bicyclo(3,2,1))-3-trioctyl
A technology of diphenylacetic acid and methyl diphenylglycolic acid, which is applied in the field of drug synthesis, can solve the problems of long reaction time, high equipment requirements, and high risk, and achieve short reaction time, low equipment requirements, and low environmental pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of Diphenylglycolic Acid
[0037] Weigh 2g of dibenzoyl into a 100ml three-necked flask, then measure 5ml of water and 5ml of absolute ethanol into the three-necked flask, and stir to dissolve the dibenzoyl. Slowly add saturated NaOH solution (containing 1.5g NaOH) dropwise in a water bath at 80°C while heating, and stop heating after reflux for 30 minutes after dropping. Cool, add 6% hydrochloric acid solution dropwise to the beaker with stirring until pH = 2-3, filter with suction, dissolve the solid with 10% NaOH solution, add activated carbon for decolorization for 5 minutes, and then filter with suction. Slowly add 6% hydrochloric acid solution dropwise to the filtrate until pH=2-3, filter the precipitated white precipitate with suction, and dry to obtain 2.1 g of the compound with a yield of 97%. Melting point: 149~151℃.
Embodiment 2
[0038] Example 2 Preparation of methyl diphenyl glycolate
[0039] Weigh 16g (0.07mol) of diphenylglycolic acid into a 250ml three-neck flask, add 100ml of methanol and stir to dissolve the diphenylglycolic acid. Add 10ml of DMC and 1ml of DBU, and place in a microwave reaction device for reflux reaction for 15 minutes. After the reaction, recover most of the methanol (about 95% by volume), cool the distillate and transfer it to a 250ml beaker, add distilled water 3 times the volume of the distillate to the beaker under stirring, and adjust the pH value of the solution to 9-10, a white solid is produced during the process, the solid is filtered out with suction, and dried at 50°C to obtain the crude methyl diphenylglycolate. Crude product with V 乙酸乙酯 :V 石油醚 =1:3.5 The mixed solution was recrystallized, filtered and dried to obtain 15.24 g of compound, with a yield of 90%. Melting point: 68~70℃.
Embodiment 3
[0040] Example 3 Preparation of Tropin Diphenylglycolate
[0041] Weigh 2.8g (0.02mol) of tropinol into a 250ml three-neck flask, add 60ml of fresh anhydrous toluene, and slowly distill half of the solvent using a distillation device. Then take 1.3g of sodium methoxide and 6g (0.025mol) of methyl diphenylglycolate into the three-necked flask, add 70ml of fresh anhydrous toluene, and reflux for 15h under stirring. After the reaction, put the reaction solution in an ice-water bath to cool to below room temperature, add 40ml of ice water while stirring, and then stir again until solid precipitates. Put the reaction solution in the refrigerator to cool and crystallize for 24 hours, and the precipitated solid was washed twice with 5ml of cold anhydrous toluene to obtain a crude product of tropine diphenylglycolate, which was then recrystallized to obtain 4.2 g of a white solid, with a yield of 60% . Melting point 146-148°C, mass spectrometry m / e: [M+1]+352.3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com