Cultural method for inducing human embryonic stem cell to directionally differentiate into corneal limbal stem cell
A technology of corneal limbal stem cells and human embryonic stem cells, which is applied in the direction of non-embryonic pluripotent stem cells, artificially induced pluripotent cells, animal cells, etc., can solve the problems of low induction efficiency and inability to meet the clinical treatment of ocular surface diseases, and achieve reduction The effect of scar formation, repair of damaged ocular surface, and reduction of inflammatory response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Inducing Human Embryonic Stem Cells to Differentiate into Limbal Stem Cells and Preparation of Corneal Grafts
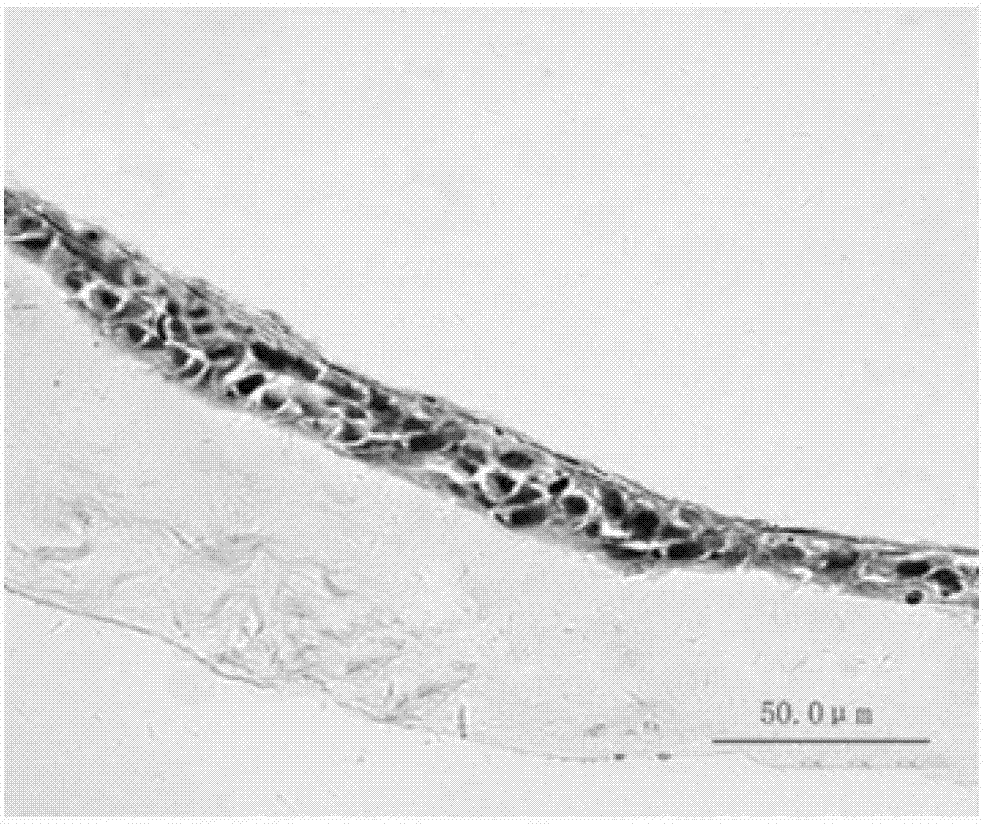
[0026] The fresh human amniotic membrane was rinsed and disinfected with PBS balanced salt solution containing 100 U / ml penicillin-streptomycin, digested with 0.25% trypsin at 37°C for 30 minutes, and the amniotic membrane cells were removed by pipetting to obtain the decellularized amniotic membrane scaffold material. The acellular amniotic membrane scaffold material was cut into discs with a diameter of 3 cm using ophthalmic scissors under a dissecting microscope. Human embryonic stem cells were cultured in induction medium for 9 days to differentiate into limbal stem cell-like cells (such as figure 1 b) Digest induced cells with 0.125% trypsin at 37°C for 5 minutes to prepare single cell suspension, press 1.5X10 4 cells / cm 2 Inoculate it on the cut acellular amniotic membrane scaffold material (the inoculation area of the scaffold material is ab...
Embodiment 2
[0032] The tissue-engineered ocular surface constructed in Example 2 repairs chemically damaged corneal epithelium
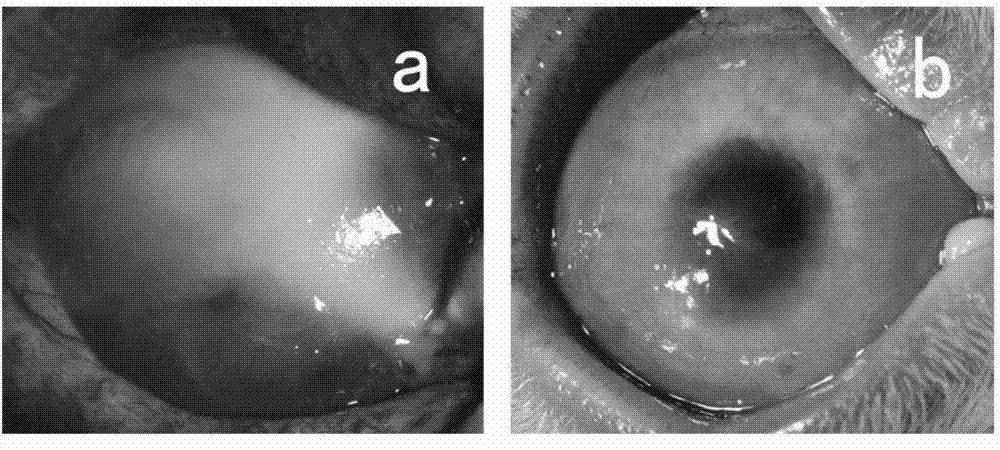
[0033] Soak the ring-shaped filter paper in 1mol / L soda ash for 20 seconds, absorb the excess liquid with sterile gauze, place it on the ocular surface of the rabbit and cauterize it for 40 seconds, then rinse the ocular surface with a large amount of sterile saline to prepare rabbit limbal stem cell degeneration compensation model. One month after the burn, select the successfully constructed rabbit corneal limbal stem cell decompensation model, carefully remove the new blood vessel fibrous membrane under the microscope, clean the implant bed, and place the induced cell graft (prepared in Example 1) on the prepared implant bed. Suture the edges of the graft with 10-0 nylon sutures. The implants were observed under the slit lamp every 3 days after the operation, and the cell growth was observed under the confocal microscope every 1 month. Three months after tr...
Embodiment 3
[0034] The tissue-engineered ocular surface constructed in Example 3 repairs mechanically damaged corneal epithelium
[0035] The rabbit corneal limbal tissue was excised circularly under a microscope with a lamellar knife to prepare a rabbit corneal limbal stem cell decompensation model. One month after the surgical injury, select the successfully constructed rabbit corneal limbal stem cell decompensation model, carefully remove the new blood vessel fibrous membrane under the microscope, clean the implant bed, and place the induced cell graft (prepared in Example 1) on the prepared implant bed , with 10-0 nylon suture at the edge of the graft. The implants were observed under the slit lamp every 3 days after the operation, and the cell growth was observed under the confocal microscope every 1 month. Three months after transplantation, the experimental animals were sacrificed for histological detection, such as Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com