Staphylococcal enterotoxin A chemiluminiscence enzyme-linked immunoassay detection kit
A staphylococcal enteric and chemiluminescent enzyme technology, which is applied in the direction of chemiluminescence/bioluminescence, analysis of materials through chemical reactions, and analysis of materials, can solve the problems of many interference factors, expensive, time-consuming, etc., and achieve repeatability Good, strong specificity, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
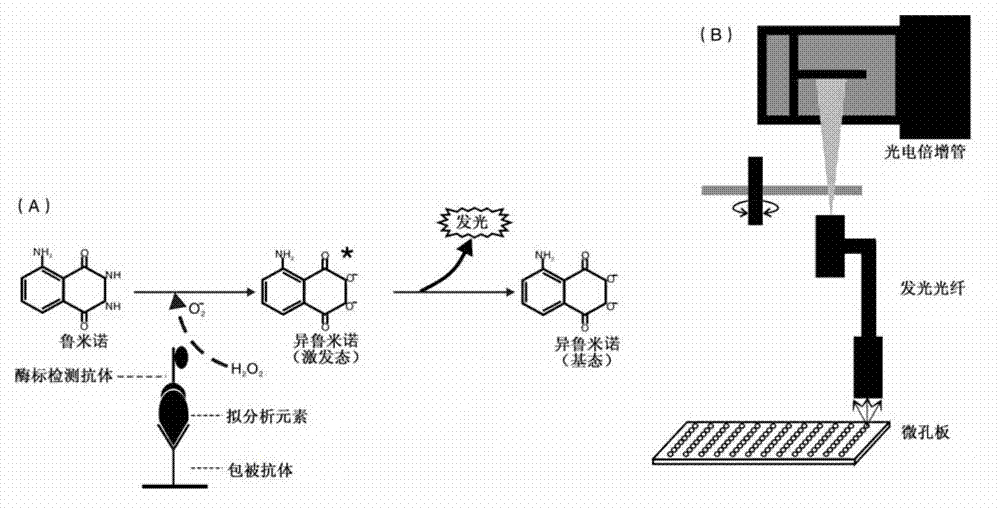
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of Staphylococcus aureus Enterotoxin A Monoclonal Antibody and Screening of Coating Antibody and Detection Antibody Pairing
[0046] (1) Preparation of monoclonal antibody against Staphylococcus aureus enterotoxin A: The purified natural Staphylococcus aureus enterotoxin A antigen standard was provided by the Institute of Microbial Epidemiology, Academy of Military Medical Sciences. Eight-week-old BALB / c mice were used as immunized animals. Staphylococcus aureus enterotoxin A was used as the immunogen, and the first immunization dose was 50 μg / mL. The immunogen was dissolved in normal saline and an equal volume of Freund’s complete adjuvant. Emulsifier, multi-point subcutaneous injection. The second immunization was carried out after an interval of 3 weeks. The dose and route were the same as the first immunization, and the adjuvant was Freund's incomplete adjuvant. After 2-3 weeks, the third immunization was carried out, the dose was the same as...
Embodiment 2
[0053] Example 2: Establishment of Chemiluminescent ELISA Method
[0054] (1) Selection of optimal coating antibody concentration: Coating antibody is coated with chemiluminescent enzyme at serial dilutions of 1.25 μg / mL, 2.5 μg / mL, 5 μg / mL, 10 μg / mL, 20 μg / mL and 40 μg / mL Connect plates, 100 μL / well, incubate overnight at 4°C, wash the plate 3 times with washing solution; seal with 250 μL / well blocking solution, place at room temperature for 1 hour, wash the plate 3 times; add 0.01ng / mL Staphylococcus aureus enterotoxin A Standard antigen, 100 μL / well, at the same time each coating antibody concentration was used as a blank control well, incubated at 37°C for 1 hour, and washed 3 times; respectively added 1:4000 diluted anti-Staphylococcus aureus enterotoxin A detection antibody, 37 Incubate at ℃ for 1 hour, wash the plate 5 times; add 100 μL / well of chemiluminescence solution, and measure the luminescence value. According to the ratio of the measured different coating antib...
Embodiment 3
[0064] Example 3: Application of chemiluminescent ELISA detection kit for detection of Staphylococcus aureus enterotoxin A in different matrices
[0065] This example is used to evaluate the accuracy and practicability of the staphylococcal enterotoxin A chemiluminescent ELISA detection kit of the present invention in detecting staphylococcus aureus enterotoxin A in different matrices.
[0066] Dilute the standard Staphylococcus aureus enterotoxin A with environmental matrix river water, food matrix milk, body fluid matrix human serum or human urine to different concentrations, detect the content of Staphylococcus aureus enterotoxin A by chemiluminescent enzyme immunoassay, and calculate The ratio of the measured concentration to the actual concentration added is the recovery rate.
[0067] (1) Coat the chemiluminescence enzyme-linked plate with the coating antibody: Dilute the coating antibody to 2.5 μg / mL with the coating solution and add to the chemiluminescence enzyme-link...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com