Cyclo-metalated iridium-azo complex as well as preparation method and application thereof
A technology of cyclometalation and complexes, which is applied in the field of preparation of cyclometalated iridium complexes, can solve the problems of self-quenching of endogenous fluorescent dyes, unfavorable imaging stability, and influence of cell observation, etc., and achieve background fluorescence intensity Weak, simple and stable results, high dyeing sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
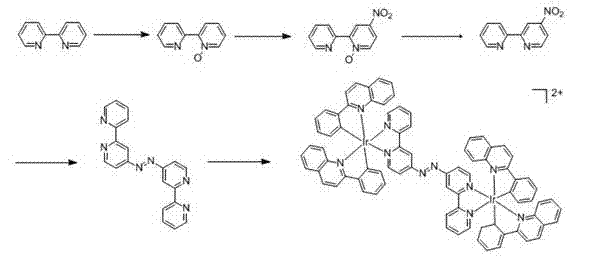
[0023] Example 1 Preparation of Ligand Selenium-5,6-dione-1,10-phenanthroline (phendione-Se)
[0024] (1) 2,2'-bipyridine-N-oxide
[0025] You can refer to the literature ( Chem. Comm. 2011, 47 , 11011-11013) method to prepare. Dissolve 7.8 g of 2,2’-bipyridine in 40 mL of trifluoroacetic acid, add 8.5 mL of 30% hydrogen peroxide, stir at room temperature for 4 h, neutralize with 6 N sodium hydroxide solution, and then use 4′ 50 cm 3 After extraction with chloroform, the organic phases were combined and washed with saturated sodium chloride solution, then dried over anhydrous sodium sulfate, filtered and evaporated to remove chloroform to obtain a colorless oil, which was dried overnight in vacuum to obtain a white needle-like solid.
[0026] (2) 4-Nitro-2,2'-bipyridine-N-oxide
[0027] Dissolve 3 g of 2,2'-bipyridine-N-oxide in 19 mL of concentrated sulfuric acid with stirring in an ice bath, then add dropwise to fuming nitric acid / concentrated sulfuric acid (30 mL / 14...
Embodiment 2
[0036] Example 2 [Ir(pq) 2 (azobpy)Ir(pq) 2 ] 2+ In Vitro Response Experiments to Physiological Thiols
[0037] The complexes used below are the compounds prepared in Example 1.
[0038] Prepare a 5 mM complex solution with acetonitrile / HEPES (10 mM, pH 7.5) buffer solution with a volume ratio of 1:1, add different concentrations of cysteine, homocysteine or glutathione dropwise, fully After stirring, let it stand at room temperature for 5 min, and then measure its fluorescence intensity. The excitation wavelength and maximum emission wavelength of the complex are 430, 560 nm, respectively. See the experimental results image 3 . It can be seen from the figure that the original fluorescence of the complex is relatively weak, but after adding a small amount of physiological thiols, such as cysteine, homocysteine or glutathione, the fluorescence of the complex is greatly enhanced. Has a very sensitive fluorescence response.
Embodiment 3
[0039] Example 3 [Ir(pq) 2 (azobpy)Ir(pq) 2 ] 2+ Intracellular Response Experiments to Physiological Thiols
[0040] The complexes used below are the compounds prepared in Example 1.
[0041] Cell culture: Hela cells were cultured in DMEM medium containing 10% fetal bovine serum, cells (5′ l0 8 / L) inoculated in a confocal microscope special glass-bottom culture dish, the diameter of the culture dish is 35 mm, the thickness of the cover glass is 0.085~0.13 mm, the diameter of the micropore in the center of the dish is 10 mm, and 5% CO 2 Incubate at 37°C under 95% air conditions, and after 24 hours of adherent growth, replace with serum-free medium and continue to incubate for 4 hours.
[0042] Confocal microscope-cell imaging: Hela cells were incubated with the complex (5 μM) for 30 minutes, the culture solution was aspirated, and then washed with PBS buffer for 3 to 4 times, imaged on a Leica TCS SP5 laser scanning confocal microscope, using a 63′ / 1.4 Oil objective, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com