Organic amino compounds serving as immunopotentiators, metabolic enhancers or roborants and preparation method and use thereof
The technology of an immune enhancer and compound is applied in the field of organic amine compounds and their preparation to achieve the effects of improving disease resistance, enhancing specific and non-specific immunity of the body, and promoting the decomposition and utilization of muscle glycogen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The structure of test drug isopropyl n-butylamine is as follows:
[0038]
[0039] In Vitro Perfusion Experiment of Frog Heart and Rat Heart
[0040] Test drug: organic amine salt formed from isopropyl n-butylamine and p-acetamidobenzoic acid at a ratio of 1:1 (molar ratio, the same below).
[0041] Frog heart and mouse heart were perfused in the isolated ventricle, and the pulsation of the ventricular muscle was recorded with a multi-channel physiological recorder. 0.05% organic salt solution had a cardiotonic effect, and the cardiotonic effect was more obvious as the concentration of the test solution increased, and the high Low concentration (5%) is also safe for the heart and does not inhibit the beating of the heart.
[0042] Experiments on motility of isolated rabbit intestine
[0043] The test drug: the organic amine salt of isopropyl n-butylamine and p-acetamidobenzoic acid 1:1.
[0044]Prepare isolated rabbit intestines, place them in a Maxwellian bath cu...
Embodiment 2
[0064] Synthesis of 1-diethylamino-2-propanol:
[0065]
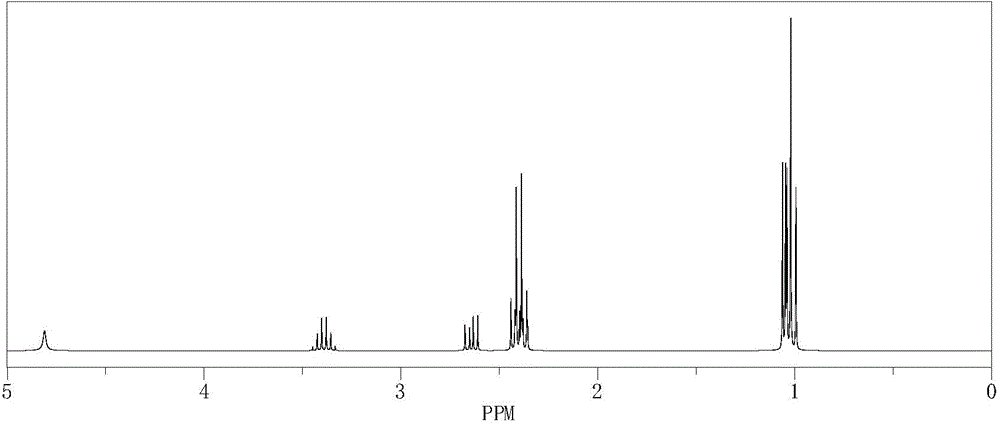
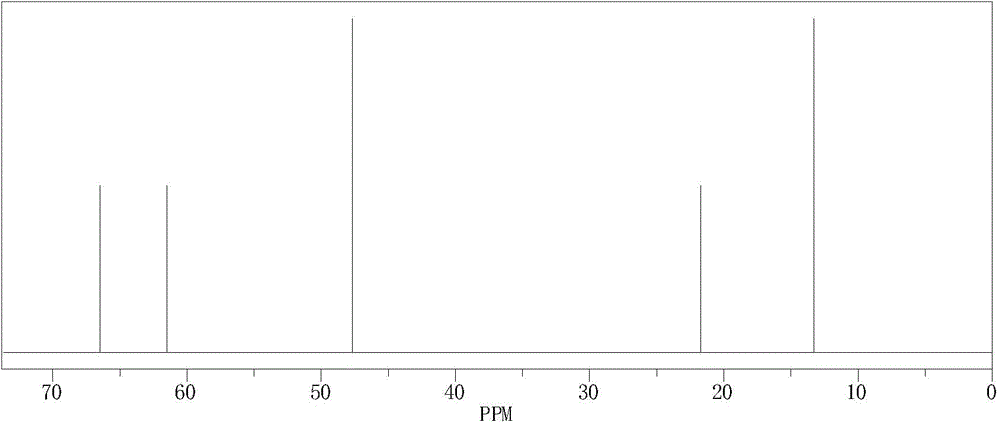
[0066] Synthetic method: Add 0.2mol of diethylamine to a 250mL four-neck flask equipped with a stirrer, reflux condenser and two dropping funnels, slowly add 0.1mol of 1-chloro-2-propanol dropwise under heating in a water bath , and then dropwise added 20ml of 40% aqueous sodium hydroxide solution, and controlled the total dropping time for 2h, then boiled and refluxed for 1h, and cooled to obtain a crude product. Sodium chloride was filtered off, the filtrate was allowed to stand for layers, and the aqueous and organic phases were separated. The aqueous phase was extracted three times with ether, and the extract was combined with the organic phase and dried overnight. Filter off the desiccant and distill to obtain the product.
[0067] refer to figure 1 and figure 2 , structure data: 1 H-NMR (400MHz, CDCl 3 )δ1.0 (m, 9H) 2.4 (t, 4H) 2.6 (d, 1H) 3.4 (m, 1H) 4.8 (s, 1H); 13 C-NMR (400MHz, CDCl 3 ) δ 13.3 (2C) ...
Embodiment 3
[0069] Synthesis of isopropyl n-butylamine:
[0070]
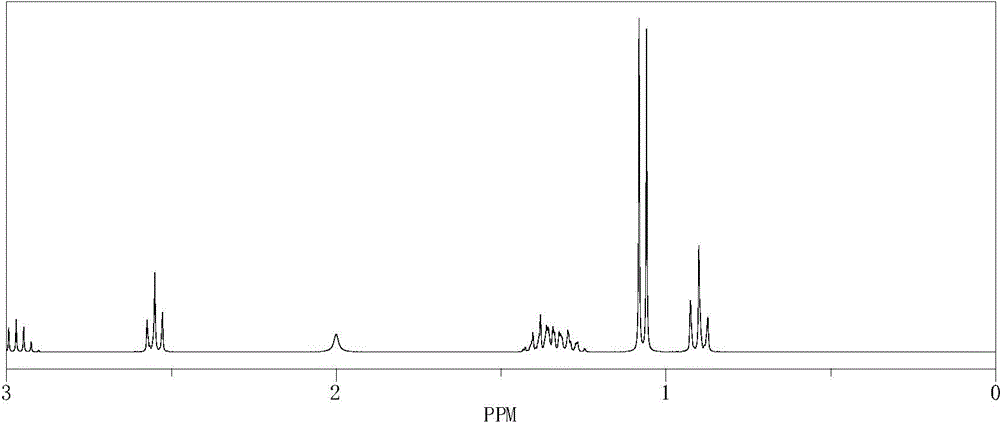
[0071] Synthesis method: Add 0.2mol of isopropylamine to a 250mL four-neck flask equipped with a stirrer, reflux condenser and dropping funnel, slowly add 0.1mol of 1-bromobutane dropwise under heating in a water bath, and then dropwise add Add 20ml of 40% sodium hydroxide aqueous solution, control the total dropping time for 2 hours, then boil and reflux for 1 hour, and cool to obtain the crude product. Sodium chloride was filtered off, the filtrate was allowed to stand for layers, and the aqueous and organic phases were separated. The aqueous phase was extracted three times with ether, and the extract was combined with the organic phase and dried overnight. Filter off the desiccant and distill to obtain the product.
[0072] refer to image 3 and Figure 4 , structure data: 1 H-NMR (400MHz, CDCl 3 )δ0.9(t, 3H) 1.1(d, 6H) 1.3(m, 4H) 2.0(s, 1H) 2.5(t, 2H) 3.0(m, 1H); 13 C-NMR (400MHz, CDCl 3 ) δ 13.8 (1C) 20.1 (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com