Method for preparing cyan aromatic hydrocarbon by using aryl bromide
A technology of cyanoarenes and brominated arenes, which is applied in pharmaceutical and chemical intermediates and related chemical fields, can solve the problems of harsh reaction conditions and high reaction temperature, and achieve the effect of short synthesis route, few reaction steps and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
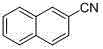
[0020] Accurately weigh p-bromoacetophenone (99.0 mg, 0.5 mmol), potassium ferrocyanate trihydrate (84.5 mg, 0.2 mmol), palladium acetate (5.6 mg, 0.025 mmol), N, P - Bidentate ligand (12.9 mg, 0.025 mmol), potassium carbonate (138.0 mg, 1.0 mmol) were successively added to a 25 mL Schlenk bottle, acetonitrile (2.0 mL) was added, and placed in a 100 oC oil bath for 24 h . After the reaction, the reaction solution was filtered and spin-dried, and separated through a silica gel chromatography column, and the yield of p-cyanoacetophenone (a) was 85%. 1 H NMR (400 MHz, CDCl 3 ) δ 8.03 (d, J = 8.5 Hz, 2H), 7.77 (d, J = 8.5 Hz, 2H), 2.63 (s, 3H). 13 C NMR (100 MHz, CDCl 3 ) δ 196.55, 139.92, 132.53, 128.71, 117.94, 116.41, 26.78.
[0021]
[0022] (a)
Embodiment 2
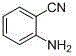
[0024] Accurately weigh o-bromoaniline (85.5 mg, 0.5 mmol), potassium ferrocyanide trihydrate (84.5 mg, 0.2 mmol), palladium acetate (5.6 mg, 0.025 mmol), N, P - Bidentate ligand (12.9 mg, 0.025 mmol), potassium tert-butoxide (112.3 mg, 1.0 mmol) were successively added to a 25 mL Schlenk bottle, acetonitrile (2.0 mL) was added, and placed in a 100 oC oil bath for reaction 48 h. After the reaction, the reaction solution was filtered and spin-dried, and separated through a silica gel chromatography column, and the yield of p-2-aminobenzonitrile (b) was 62%. 1 H NMR (400 MHz, CDCl 3 ) δ 7.38 (dd, J = 8.0, 1.3 Hz, 1H), 7.35 – 7.29 (m, 1H), 6.78 – 6.68 (m, 2H), 4.41 (s, 2H). 13 C NMR (100 MHz, CDCl 3 ) δ 149.53, 133.97, 132.32, 117.96, 117.58, 115.09, 95.98.
[0025]
[0026] (b)
Embodiment 3
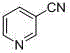
[0028] Accurately weigh p-bromoaniline (85.5 mg, 0.5 mmol), potassium ferrocyanide trihydrate (84.5 mg, 0.2 mmol), palladium acetate (5.6 mg, 0.025 mmol), N, P - Bidentate ligand (12.9 mg, 0.025 mmol), sodium carbonate (106.0 mg, 1.0 mmol) were successively added to a 25 mL Schlenk bottle, acetonitrile (2.0 mL) was added, and placed in a 100 oC oil bath for 48 h . After the reaction, the reaction solution was filtered and spin-dried, and separated through a silica gel chromatography column, and the yield of p-aminobenzonitrile (c) was 78%. 1 H NMR (400 MHz, CDCl 3 ) δ 7.40 (d, J= 8.7 Hz, 2H), 6.64 (d, J = 8.7 Hz, 2H), 4.20 (s, 2H). 13 C NMR (100 MHz, CDCl 3 ) δ 150.41, 133.71, 120.14, 114.34, 99.93.
[0029]
[0030] (c)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com