Human enterovirus four-color fluorescence RT-PCR detection kit and detection method
A technology of RT-PCR and detection kits, which is applied in the field of four-color fluorescent RT-PCR detection kits, can solve the problems of cumbersome detection steps, low specificity and sensitivity, save production costs and detection costs, and avoid specificity Not high, time-shortening effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0051] Enterovirus four-color fluorescent RT-PCR kit
[0052] 1. RT-PCR kit composition
[0053] This kit includes multiple fluorescent RT-PCR reaction master solution, positive control substance, negative control substance, internal control, AMV reverse transcriptase, Taq polymerase. The multiple fluorescent RT-PCR reaction master solution contains multiple 10× fluorescent RT-PCR reaction buffer, EV universal primers, EV universal probes, EV71 primers, EV71 probes, CoxA16 primers, CoxA16 probes, internal Control primers, internal control probes, bovine serum albumin; multiplex 10× fluorescent RT-PCR reaction buffer contains tris hydrochloride, potassium chloride, glycerin, four kinds of mononucleotide monomers, and magnesium chloride.
[0054] The multiple fluorescent RT-PCR reaction master solution is prepared as follows (the corresponding substance concentrations are shown in brackets):
[0055] Multiplex 10×PCR reaction buffer 5ul
[0056] Bovine serum albumin (20mg / ml)...
specific Embodiment 2
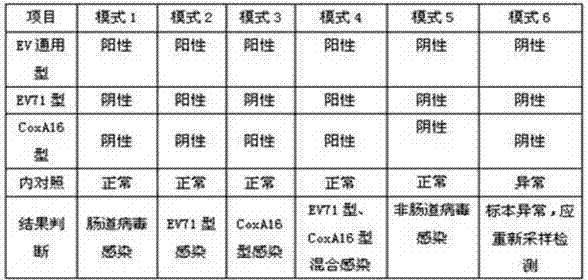
[0088] Four-color fluorescent RT-PCR detection method for human enterovirus, positive control and negative control should be set up for each detection
[0089] 1. Sample processing:
[0090] Collect throat swabs, herpes fluid, cerebrospinal fluid or human enterovirus isolation culture from suspected patients, and quickly put them into a commercial sampling tube (Youkang Jiye, MT0301-1) containing 1-2ml of virus preservation solution as the sample to be tested use;
[0091] 2. Extraction of viral nucleic acid RNA:
[0092] Strictly follow the commercial nucleic acid RNA extraction kit QIAGEN Viral RNA Mini Extraction Kit (QIAGEN, CAT: 52904) to extract viral RNA from clinical specimens or virus isolation cultures; add 10ul internal control (concentration: 10 4 copies / ml) to participate in the extraction of nucleic acid samples simultaneously;
[0093] 3. RT-PCR reaction system preparation
[0094] Take 10ul extracted sample nucleic acid, 10ul positive control substance and ...
specific Embodiment 3
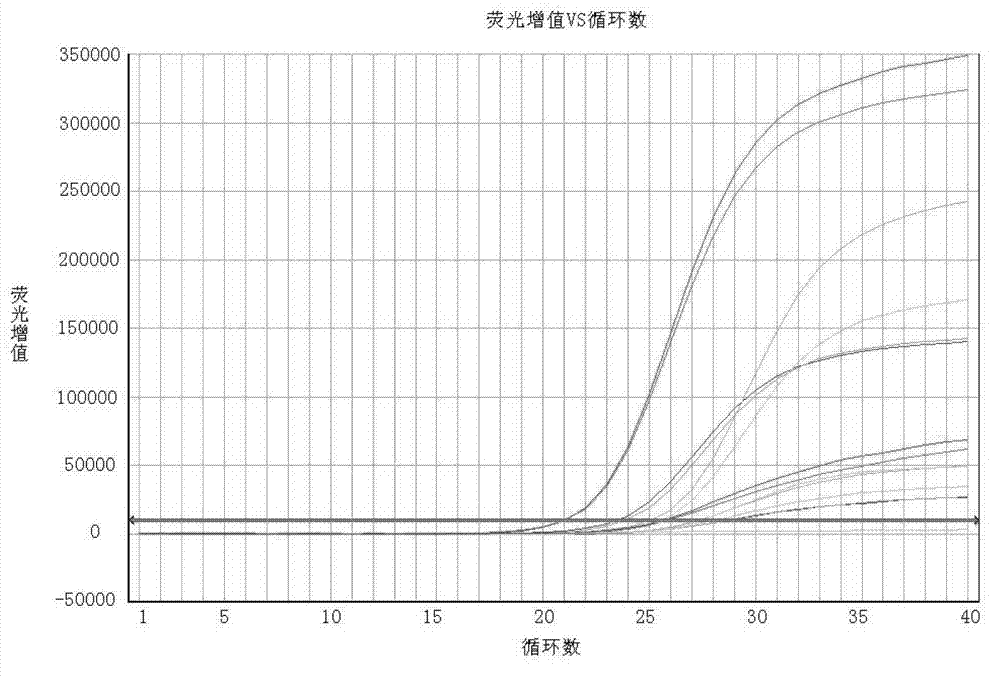
[0105] Detection Kit Sensitivity and Specificity Analysis
[0106] 1. Experimental material virus strain
[0107] Target strain group: Enterovirus EV71 strain, Coxsackie virus group A type 16 strain, Coxsackie virus group A type 1, Coxsackie virus group A type 11, Coxsackie virus group A type 13 , Coxsackie virus group B type 3, enterovirus EV68 type, echovirus type 5, echovirus type 13; non-target strain group: rotavirus, norovirus type I and II, type A H1N1 Influenza virus and influenza B virus, the above-mentioned strains were purchased from the American Culture Collection and Management Center, or isolated and preserved by the Ningbo Center for Disease Control and Prevention.
[0108] 2. Test results
[0109] Enterovirus four-color fluorescent RT-PCR kit was used to detect the above strains, and the target strain group included Enterovirus EV71 strain, Coxsackievirus A group 16 strain, Coxsackievirus A group 1 Coxsackievirus A group 11, Coxsackievirus A group 13, Coxsac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com